CBSE Class 12-science Answered
Which of the following molecular orbital has two nodal planes ?
(1)
π 2Py
(2)
σ 2s
(3)
π* 2Py
(4)
σ* 2Pz
Asked by Atulcaald | 25 May, 2018, 12:34: AM
The number of orbitals whose number of node planes are as follows:
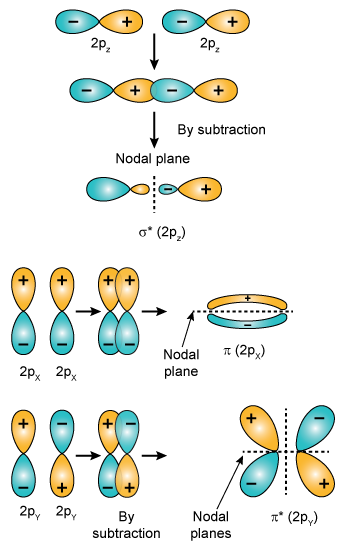
The nodal planes are σ 1s = σ 2px = 1, π 2pz = 1 , π*2py = 2
Answered by Ramandeep | 25 May, 2018, 09:48: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by ukg8612 | 15 Apr, 2024, 07:36: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by navadeepnavadeep242 | 19 Mar, 2024, 08:56: PM
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by hanihope27 | 01 Mar, 2024, 08:33: PM
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by sagarmishra | 27 Feb, 2024, 04:01: PM
CBSE 12-science - Chemistry
Asked by yashwanthgowdakn4 | 22 Feb, 2024, 09:14: PM












