CBSE Class 12-science - Batteries Videos
Batteries
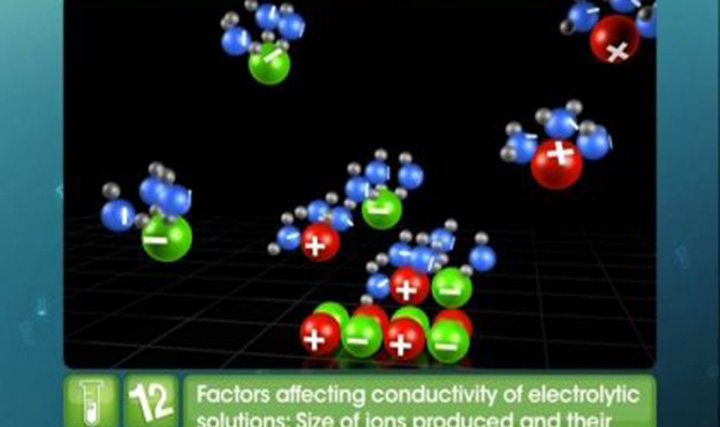
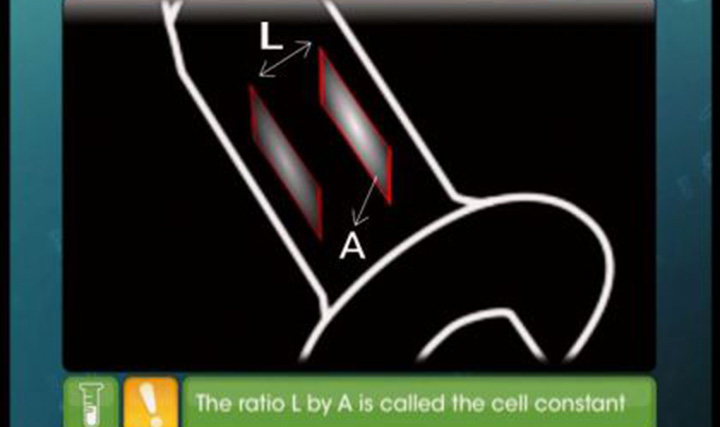
This video explains classification of cell as primary and secondary, construction, working and application of dry, mercury, fuel, lead storage and Nickel cadmium cell
More videos from this chapter
View All- Give one major advantage and disadvantage of Lead storage battery.
- Write the cell reactions which occur in lead storage battery (i) when the battery is in use (ii) when the battery is on charging.
- Which cell is a device to convert the Chemical energy of hydrogen gas into electrical energy?
- Write down the reactions taking place at the electrodes of a Mercury cell. At Anode: The Oxidation of Zinc metal takes place and the Zinc ions react with the OH- ions from the electrolyte to form Zinc hydroxide.
- What are the advantages of a fuel cell over mercury cell?
- Give a one major difference between a primary cell and a secondary cell.
- Giving Cathode and anode reaction of Nickel Cadmium cell illustrate the overall cell reaction.
- Why a Dry cell is termed as dry Cell?
- What do you understand by the term "Corrosion of dry Cell"?
- A lead storage cell acts both as Electrolytic cell as well as Galvanic cell. Explain.