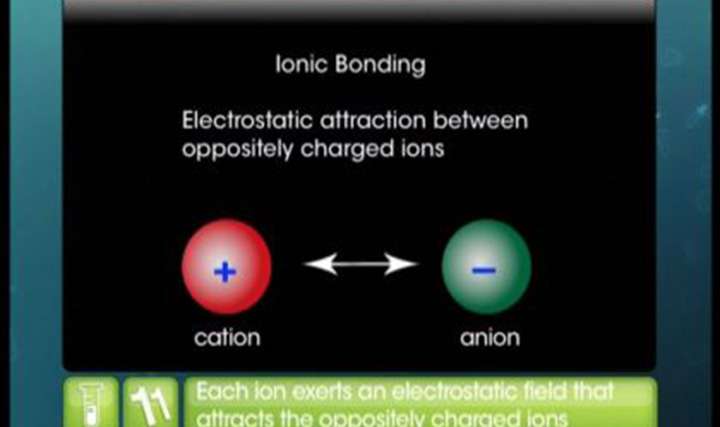
CBSE Class 11-science Chemistry Ionic Bond
-
pls solve it sir
- Give the order of melting points among NaF, MgO, Sc N, TiC.
-
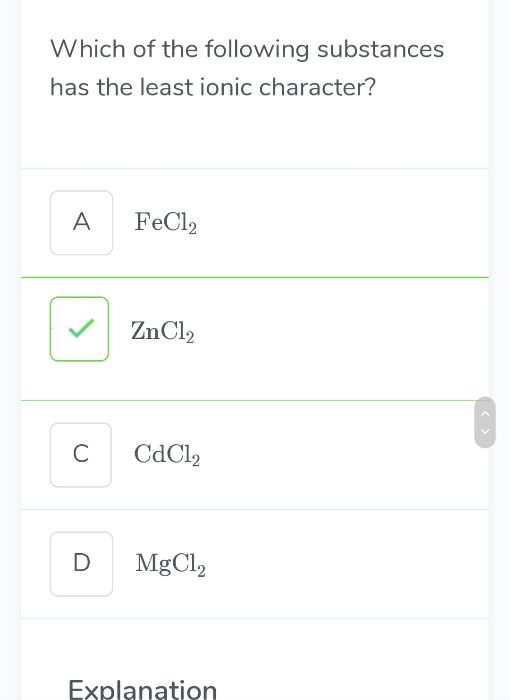
Which of the following is most ionic? Explain in detail

- A diatomic molecule has dipole moment of 1.92D and bond length of 2A. Calculate the percentage ionic character of the molecule.
- How is ionic bond formed?
- Ionic compounds do not conduct electricity when solid. Why? When do they conduct electricity?
- What is lattice energy? Which compound NaCl or MgO has higher lattice energy and why?
- Explain why reactions involving covalent compounds are generally slow?
- Which molecule among the following is most likely to have a dipole moment? CS2, SO3, H2S and SnCl4. Give reasons for your answer.
- What type of bond is present in NH3? Explain in brief.