IUPAC Nomenclature: A Comprehensive Guide for Chemistry Enthusiasts
Review the quick, simplified guide for IUPAC naming of organic compounds. Understand hydrocarbons and the rules involved in how to prioritise prefixes, add suffixes and give the compound its name.
By Topperlearning Expert 08th Jan, 2024 | 05:53 pm
ShareWith more than millions of organic compounds existing in nature, their common name-based identification is challenging. Additionally, there is a need for universal naming to avoid boundaries in research. IUPAC, or the International Union of Pure and Applied Chemistry, has been in charge of naming the elements since 1970. It eliminates cramming up the names while providing a minimal set of rules to help in the systematic naming of the compounds by any individual.
TopperLearning brings you this quick, comprehensive guide to help you understand the names of various types of organic compounds.
What are Hydrocarbons?
Breaking the term hydrocarbon, we get
Therefore, hydrocarbons are compounds that contain only hydrogen and carbon.
They may consist of straight chains, branched chains, or rings. Aromatic hydrocarbons are a particular type of cyclic hydrocarbons comprising delocalised pi electrons.
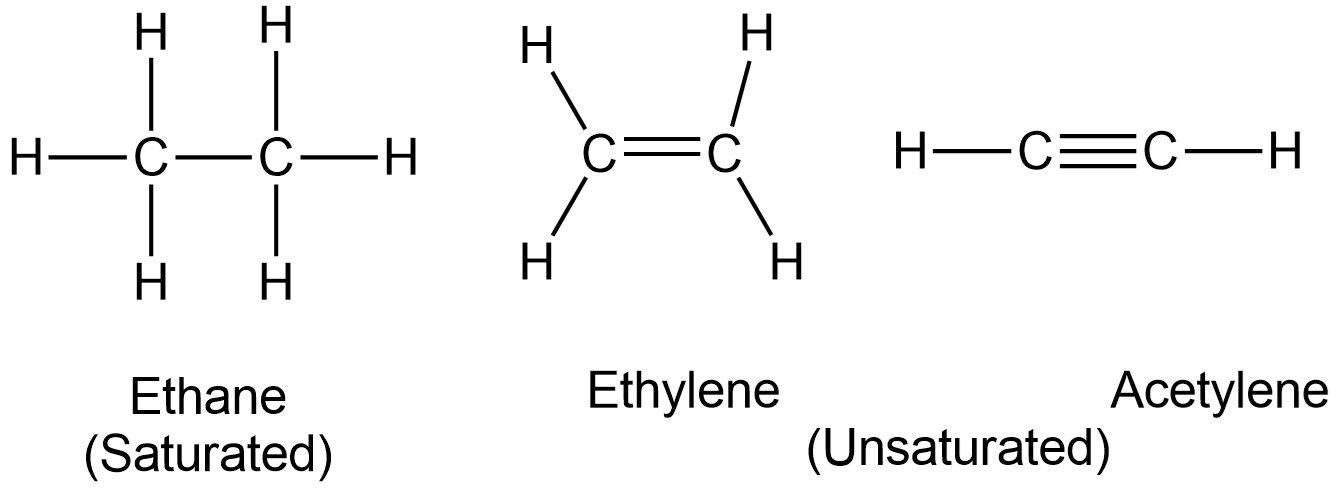
Also, depending on the covalent bonds, they can be
- Saturated hydrocarbons (C atoms linked together by single covalent bonds)
- Unsaturated hydrocarbons (C atoms linked together by double or triple bonds)
Priority Rules to Name Straight-Chain Organic Compounds
According to IUPAC, the names of compounds consist of four parts arranged as follows.
Word root
It represents the number of carbon atoms in the parent chain (longest possible continuous chain).
|
Chain Length |
Word Root |
Chain Length |
Word Root |
|
C1 |
meth |
C6 |
hex |
|
C2 |
eth |
C7 |
hept |
|
C3 |
prop |
C8 |
oct |
|
C4 |
but |
C9 |
non |
|
C5 |
pent |
C10 |
dec |
Suffix
It represents the multiple bonds and the main functional group present in the compound. So, the suffix can be:
- Primary: representing saturation or unsaturation of carbon atom in the parent chain.
|
Bond |
Suffix |
|
Single bond |
-ane |
|
Double bond |
-ene |
|
Triple bond |
-yne |
Also, if the parent carbon chain contains more than one double or triple bond, the number can be indicated by adding a numerical prefix to the primary suffix:
○ di for two
○ tri for three
○ tetra for four
-
Secondary: representing the nature of the principal functional group (when an organic compound has more than one functional group, the functional group occupying a higher position on the seniority table is the principal functional group).
|
Class of Organic Compounds |
Functional Group |
Secondary Suffix |
|
Alcohol |
-OH |
-ol |
|
Thioalcohol |
-SH |
-thiol |
|
Aldehyde |
-CHO |
-al |
|
Ketone |
-CO- |
-one |
|
Carboxylic acid |
-COOH |
-oic acid |
|
Acid chloride |
-COCI |
-oyl chloride |
|
Amide |
-CONH2 |
-amide |
|
Ester |
-COOR |
-oate |
|
Nitrile |
-CN |
-nitrile |
|
Amine |
-NH2 |
-amine |
Note: If the secondary suffix begins with a vowel, the terminal ‘e’ of the primary suffix is dropped.
For example:
|
Compound: |
Word root: Eth |
Primary suffix: -ane |
Secondary suffix: |
IUPAC name: |
-
Prefix
It represents the alkyl groups and functional groups in addition to the principal functional groups.
|
Functional Group |
Prefix |
Suffix |
|
Carboxylic Acid |
Carboxy- |
-oic acid |
|
Ester |
alkoxycarbonyl- |
-oate |
|
Acyl Halide |
halocarbonyl- |
-oyl halide |
|
Amide |
amido- |
-amide |
|
Nitrile |
cyano- |
-nitrile |
|
Aldehyde |
Oxo- |
-al |
|
Ketone |
keto/oxo |
-one |
|
Alcohol |
hydroxy- |
-ol |
|
Amine |
amino- |
-amine |
|
Ether |
alkoxy- |
-ether |
|
RCNR |
imino- |
|
|
RSH |
sulfhydryl- |
|
|
R2S |
alkylthio |
|
|
RNO2 |
nitro- |
|
|
RN2 |
diazo- |
|
|
RN3 |
azido- |
|
In the presence of the same functional group multiple times, prefixes ‘di’, ‘tri’, and others are used before the name of the functional group. For example, CH2(OH)CH2CH2(OH)CH3 is butan-1, 2-diol due to two alcoholic groups.
What is meant by Aromatic and Non-Aromatic Cyclic Carbon Compounds?
The compounds exist either in open-chained form or in closed cyclic structures. These cyclic structures are of two types:
-
Arenes or Aromatic compounds: Closed structures made of carbon, which may or may not have other elements in the framework. For a compound to be aromatic, it must follow four rules:
○ It must be a cyclic or closed structure. It should resemble a ring of atoms.
○ The molecule must be planar, which means all atoms must lie in the same plane.
○ The molecule must be fully conjugated, which means every atom must have available p-orbital.
○ The molecule should possess (4n + 2) pi electrons where n can be 0 or a positive integer.
-
Non-aromatic or Alicyclic compounds: Compounds without ring structure containing carbon along with elements. They don’t necessitate a continuous form of overlapping ring of p orbitals.
Priority Rules to Name Aromatic and Non-Aromatic Organic Compounds
In the following sections, we’ll learn the nomenclature of non-aromatic and aromatic compounds step by step:
Non-Aromatic/Alicyclic Compounds
Non-aromatic compounds are named by:
- Adding the prefix ‘cyclo’ to the alkane.
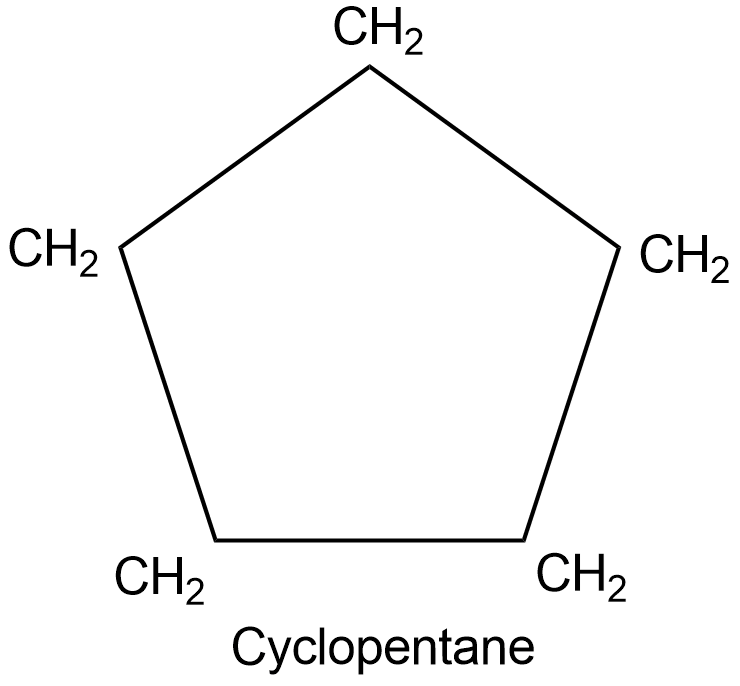
-
In the presence of side chains, the alphabetic order of numbering is used.
For example, a cyclic 6-carbon compound with methyl and propyl group will be named 1-methyl-3-propylcyclohexane.
-
In the presence of multiple bonds or functional groups, the corresponding suffixes are added to the word root. The number is done so as to give the minimum possible number to the multiple bond or functional group.
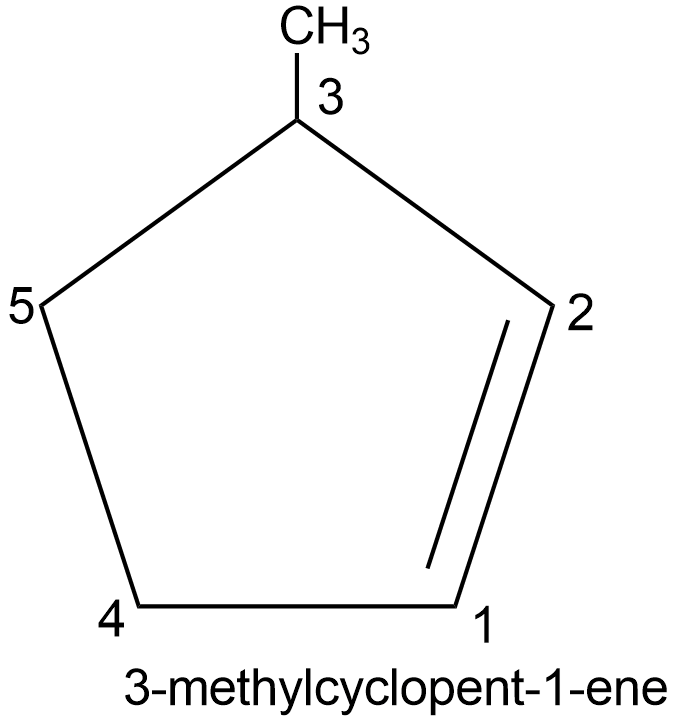
-
In the presence of a cyclic ring attached to a carbon chain:
○ If the carbon chain has more number of carbon atoms than the cyclic ring, the name will be cycloalkylalkane (e.g. 2-cyclopropylhexane)
○ If the carbon chain has a lesser number of carbon atoms than the cyclic ring, the name will be a derivative of cycloalkane (e.g. 1-propyl cyclohexane)
Arenes or Aromatic Compounds
-
The aromatic compounds with substituents are named either as ‘prefix + benzene’ or hold universal names.
-
The substituted benzene ring is numbered such that the substituent gets the lowest number.
-
In the presence of 2 substituents,
○ ortho (o) indicates compounds at 1 and 2 positions
○ meta (m) indicates compounds at 1 and 3 positions
○ para (p) indicates compounds at 1 and 4 positions
For example, the compound here will be named m-dichlorobenzene or 1, 3-dichlorobenzene.
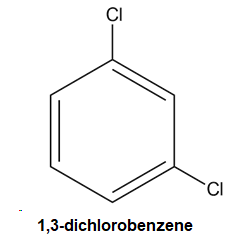
-
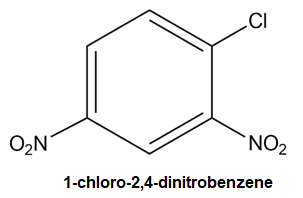
For three or more substituents, the numbering is done as per the lowest number of substituents, along with the lowest possible sum of all groups. The substitutes are listed alphabetically by name.
For example, a benzene ring with chloro and 2 nitro groups will be named 1-chloro-2, 4-dinitrobenzene.
What is Meant by the Homologous Series?
The series of compounds with common functional groups are called homologues and belong to the homologous series. The members of a specific series are represented by the general molecular formula. They differ only in the carbon chain length, i.e., two consecutive members differ by -CH2 group in their molecular formulae.
The presence of the following characteristics categorises homologous series:
-
The presence of the same functional group in all the members of the series.
-
Presence of the same general formula for hydrocarbon series.
-
Possession of the same chemical properties due to unvaried functional group.
-
The physical properties show almost regular variation.
-
The preparation method remains the same.
-
The molecular mass differs by 14 amu from that of its neighbour.
Example:
Homologues of alcohol series:
- Methyl alcohol
- Ethyl alcohol
- Propyl alcohol
- Butyl alcohol
- Pentyl alcohol
- Hexyl alcohol and so on.
Conclusion
TopperLearning- the topper’s choice online learning site brings you comprehensive study materials. Be it IUPAC from ICSE class 10 chemistry or any other topic of any class, board or subject, get every concept cleared with our expert-crafted resources.
FAQ's
Q 1. What is IUPAC nomenclature?
Ans: IUPAC nomenclature is the systematic method of naming organic chemical compounds. It is based on the rules listed to name the compounds despite their structure and isomers logically.
Q 2. How do I start naming a compound using IUPAC rules?
Ans: Begin by identifying the parent chain and assigning a root word to it. Number the chain for substituents. Name and alphabetically arrange substituents as prefixes. Next, identify and name functional groups, consider bond multiplicity, and include numbers to indicate substituent locations. If multiple functional groups are present, prioritise the one with higher naming priority.
Q 3. What are functional groups, and how do they affect naming?
Ans: Functional groups are specific groups of atoms in the molecules that impact characteristic chemical reactivity to the molecules. The numbering in the presence of functional groups is done such that the carbon attached to these gets the lowest possible number in the chain. Thus, they are determining factors in the naming of the compounds.
Q 4. How are branched compounds named?
Ans: Branched compounds are named by identifying such and numbering the longest carbon chain so that branches get the lowest numbers. The lowest number is preferred according to alphabetical order.
Q 5. How do I name cyclic compounds?
Ans: Identify the parent chain (it should be larger than the substituent) and number the substituents (if present) such that they get the lowest number. Prefer numbering in alphabetical order in the presence of multiple substituents. Ensure adding the prefix ‘cyclo’ before the corresponding straight-chain alkane.
More from Education
Important Resources
- Education Franchisee opportunity
- NCERT Solution
- CBSE Class 9 Mathematics
- NCERT Solutions for class 10 Science
- Sample Papers
- CBSE Class 9 Science
- NCERT Solutions for class 10 Maths
- Revision Notes
- CBSE Class 10 Hindi
- CBSE Class 10 English
- CBSE Class 10 English
- CBSE Class 10 Social Studies
- CBSE Class 10 Science
- CBSE Class 10 Mathematics
- Career In Science After 10
- Career In Commerce After 10
- Career In Humanities/Arts After 10
- NCERT Solutions for Class 10
- NCERT Solutions for Class 11
- Business Studies Class 12 CBSE project