CBSE Class 11-science Answered
You are provided with samples of O2,N2,CO2 and CO under similar conditions of temperature and pressure that they contain the same number of molecules represented by letter M . The molecules of O2 occupy the volume of N litres and have a mass of 8g under similar conditions of temperature and pressure
A) I) what volume is occupied by M molecules of N2?
II) what volume is occupied by 3M molecules of CO ?
B) what is the mass of CO2 in grams
C) name the law of above questions
Asked by sajidivakaran | 21 Jul, 2019, 09:41: AM
A) 1) According to avagadro's law
volume of M molecules of N2 = volume of M molecules of O2 = N litres
(under similar T and P)
2) Volume of M molecules of CO=Volume of M molecules of N2 = N litres
Volume of 3M molecules of CO= 3N litres
B)
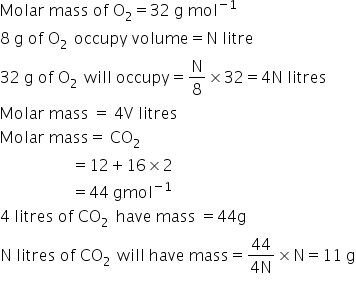
C) This law is avagadro's law.
Answered by Ravi | 22 Jul, 2019, 12:01: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mohd.arhaan812 | 06 May, 2024, 21:55: PM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 22:43: PM
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 20:58: PM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 00:09: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 14:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 22:07: PM
CBSE 11-science - Chemistry
Asked by devanshuchhipani | 12 Mar, 2020, 20:42: PM
CBSE 11-science - Chemistry
Asked by ritua7330 | 01 Sep, 2019, 12:53: PM
CBSE 11-science - Chemistry
Asked by sajidivakaran | 21 Jul, 2019, 09:41: AM
CBSE 11-science - Chemistry
Asked by shashank1854 | 28 May, 2019, 16:48: PM