CBSE Class 11-science Answered
an ideal gas mixture contains 4 milimoles of H2 for every mili mole of Ne .The partial pressure of Ne is ?

Asked by pushpakumari291279 | 31 Dec, 2020, 14:02: PM
If an ideal gas mixture contains 4 milli moles of H2 for every milli mole of Ne.
Ratio of millli moles-
H2 : Ne
4:1
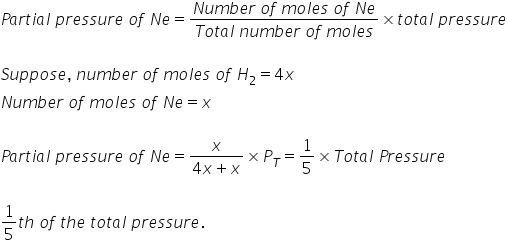
Answered by Ravi | 01 Jan, 2021, 11:43: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by mohd.arhaan812 | 06 May, 2024, 21:55: PM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 22:43: PM
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 20:58: PM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 00:09: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 14:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 22:07: PM
CBSE 11-science - Chemistry
Asked by devanshuchhipani | 12 Mar, 2020, 20:42: PM
CBSE 11-science - Chemistry
Asked by ritua7330 | 01 Sep, 2019, 12:53: PM
CBSE 11-science - Chemistry
Asked by sajidivakaran | 21 Jul, 2019, 09:41: AM
CBSE 11-science - Chemistry
Asked by shashank1854 | 28 May, 2019, 16:48: PM