CBSE Class 12-science Answered
Write the chemical equations for the preparation of KMnO4 from MnO2.
Asked by sanjeet.kumar | 12 Mar, 2019, 14:21: PM
Preparation of potassium permanganate: Potassium permanganate is prepared by the fusion of MnO2 (pyrolusite) with potassium hydroxide and an oxidizing agent like KNO3 to form potassium manganate (green mass), which disproportionate in a neutral or acidic solution to form permanganate.
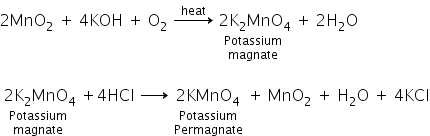
Answered by Ramandeep | 12 Mar, 2019, 19:24: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by gupta.sandhya2007 | 23 May, 2024, 08:16: AM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 24 Feb, 2020, 19:28: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:21: PM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 04 Aug, 2018, 20:20: PM
CBSE 12-science - Chemistry
Asked by minipkda | 22 May, 2018, 06:04: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 14:00: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 14:00: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 09:08: AM




 and
and  ? Write it chemical reaction ?
3) give the disproportionation reaction of
? Write it chemical reaction ?
3) give the disproportionation reaction of  4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P
4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P MOLECULE ARE NOT EQUIVALENT
MOLECULE ARE NOT EQUIVALENT 