CBSE Class 12-science Answered
1) which one of l and
and  is not likely to exist ?
2) how does ammonia react with AgN
is not likely to exist ?
2) how does ammonia react with AgN ? Write it chemical reaction ?
3) give the disproportionation reaction of
? Write it chemical reaction ?
3) give the disproportionation reaction of  4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P
4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P MOLECULE ARE NOT EQUIVALENT
MOLECULE ARE NOT EQUIVALENT
 and
and  is not likely to exist ?
2) how does ammonia react with AgN
is not likely to exist ?
2) how does ammonia react with AgN ? Write it chemical reaction ?
3) give the disproportionation reaction of
? Write it chemical reaction ?
3) give the disproportionation reaction of  4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P
4) HYPOPHOSPHURUS acis is a good reducing agent . Justify with example
5) give chemical equation in support of the statement that all bonds in P MOLECULE ARE NOT EQUIVALENT
MOLECULE ARE NOT EQUIVALENT
Asked by niharikapabba2605 | 04 Aug, 2018, 20:20: PM
1) Which one among PCl4+ and PCl4- is not likely to exist?
Ans: PCl4+ exists more likely than PCl4-.
As we move down the group the stability of +5 oxidation state decreases and the stability of +3 oxidation state increases.
PCl4+ is more stable as it has +5 which is more stable.
2) 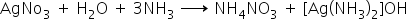
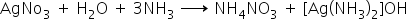
3)

4) In H3PO2, two hydrogen atoms are bonded directly to the phosphorous atom which imparts the reducing character to the acid.
5) PCl5 has trigonal pyramidal structure and the three equatorial P-Cl bonds re equivalent, while two axial bonds are different and longer then equatorial bonds.
On heating, PCl5 breaks down into phosphorous trichloride and chlorine gas, which shows that out of five P-Cl bonds three are equatorial and tightly bonded and two are axial which are weak bonds hence breaks.
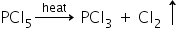
Answered by Ramandeep | 06 Aug, 2018, 14:26: PM
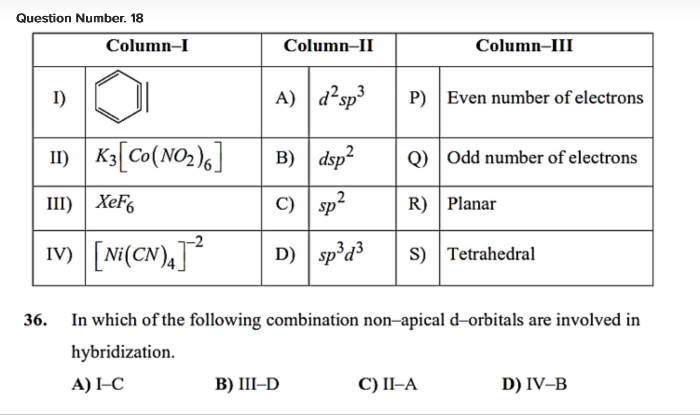
Concept Videos
CBSE 12-science - Chemistry
Asked by gupta.sandhya2007 | 23 May, 2024, 08:16: AM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 24 Feb, 2020, 19:28: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 14:21: PM
CBSE 12-science - Chemistry
Asked by niharikapabba2605 | 04 Aug, 2018, 20:20: PM
CBSE 12-science - Chemistry
Asked by minipkda | 22 May, 2018, 06:04: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 14:00: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 14:00: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2014, 09:08: AM