CBSE Class 11-science Answered
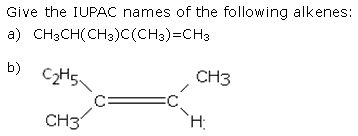
Why do alkenes show geometrical isomerism? Explain with examples.
Asked by Topperlearning User | 01 Jun, 2016, 13:50: PM
- Due to C=C bond, the rotation around this bond is restricted.
- Geometrical isomerism is due to the presence of like groups on the same side(cis-isomer) or on the opposite side (trans-isomer).
- The presence of two different groups at the doubly bonded carbon is the necessary and sufficient condition for geometrical isomerism.
C(a,b) = C(a,b) , C(a,b) = C(e,f) or
C(a,b) = C(a,d) all show geometrical isomerism.
Answered by | 01 Jun, 2016, 15:50: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 13:39: PM
CBSE 11-science - Chemistry
Asked by debupatel8899 | 06 Feb, 2020, 07:04: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:39: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:52: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:55: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 10:57: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 13:50: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 11:20: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 23 Jul, 2014, 11:28: AM