ICSE Class 10 Answered
which element has smallest atomic radius and which element has largest atomic radius in the periodic table?
Asked by saibaba1069 | 23 Apr, 2020, 00:40: AM
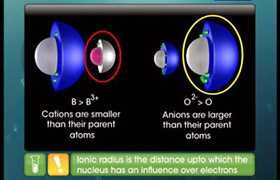
The atomic radius decreases as we move from left to right along the period. And The atomic radius increases down the group.
Thus, helium is the smallest element, and francium is the largest.
Answered by Ramandeep | 23 Apr, 2020, 10:49: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by sagarmishra | 12 Mar, 2024, 09:48: AM
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 03 Mar, 2024, 19:07: PM
ICSE 10 - Chemistry
Asked by nandu.bandhiye08091983 | 23 Nov, 2023, 22:30: PM
ICSE 10 - Chemistry
Asked by bharathijaisimha03 | 29 Sep, 2023, 07:01: AM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 18 Jul, 2022, 22:36: PM
ICSE 10 - Chemistry
Asked by bethelhouse434a | 27 Jun, 2022, 22:08: PM
ICSE 10 - Chemistry
Asked by maneesha.gangan | 10 Feb, 2022, 20:13: PM
ICSE 10 - Chemistry
Asked by ch.satyanarayana112233 | 05 Oct, 2020, 11:21: AM
ICSE 10 - Chemistry
Asked by nitushivankar706 | 07 Aug, 2020, 11:29: AM
ICSE 10 - Chemistry
Asked by saibaba1069 | 23 Apr, 2020, 00:42: AM