ICSE Class 10 Answered
What volume of oxygen at STP is required to affect the combustion of 11 litres of ethylene at 273°C and 380 mm of Hg pressure?
Answer in my book : 8.25 litres
Asked by singhamaya63 | 24 Feb, 2020, 11:50: AM
Given:
P = 380 mmHg
= 0.5 atm
T = 273 °C
= 273+273
= 546 K
V = 11 litre
No. of moles = n
We have,
PV = nRT
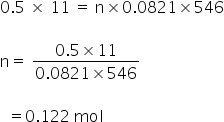 The reaction is :
The reaction is :
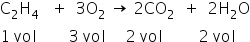 So for 1 mol of C2H4 combines with 3 mol of O2
Now 0.122 moles of C2 H4 will combine with 0.122×3
= 0.368 mol
Volume of 1 mole of gas is 22.4 litre
Volume of 0.368 mol = 22.4 × 0.368
= 8.24 litre
Volume of oxygen is 8.24 litre.
So for 1 mol of C2H4 combines with 3 mol of O2
Now 0.122 moles of C2 H4 will combine with 0.122×3
= 0.368 mol
Volume of 1 mole of gas is 22.4 litre
Volume of 0.368 mol = 22.4 × 0.368
= 8.24 litre
Volume of oxygen is 8.24 litre.
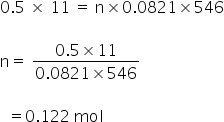
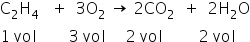
Answered by Ravi | 24 Feb, 2020, 12:40: PM
Concept Videos
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 05 Mar, 2024, 18:40: PM
ICSE 10 - Chemistry
Asked by kundus458 | 07 Feb, 2024, 08:55: AM
ICSE 10 - Chemistry
Asked by matloobser | 07 Sep, 2023, 09:36: AM
ICSE 10 - Chemistry
Asked by dafk04.dp | 06 May, 2021, 18:22: PM
ICSE 10 - Chemistry
Asked by amit.clw4 | 15 Mar, 2021, 07:27: AM
ICSE 10 - Chemistry
Asked by amit.clw4 | 14 Mar, 2021, 08:12: AM
ICSE 10 - Chemistry
Asked by ravi.solaabhi | 17 Oct, 2020, 10:11: AM
ICSE 10 - Chemistry
Asked by payalagrawal1724 | 28 Jun, 2020, 19:22: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 29 Dec, 2019, 20:07: PM
ICSE 10 - Chemistry
Asked by Dsangayy | 08 May, 2019, 19:11: PM






