CBSE Class 12-science Answered
What volume of 95% sulphuric acid (density = 1.85 g/mL) and what mass of water must be taken to prepare 100 mL of 15% solution of sulphuric acid (density = 1.10 g/mL) ?
Asked by abhinavsaini123 | 06 Jul, 2015, 09:16: AM
To find:
Volume of 95% sulphuric acid (density = 1.85 g/mL)
Mass of water to prepare 100 mL of 15% solution of sulphuric acid (density = 1.10 g/mL)
Solution:
Volume of the solution = 100 cm2
Density of the solution = 1.10 g/cm3
Therefore, Mass of 100 cm3 of solution = 100 x 1.10 g =110 g
The given solutions is 15%. Therefore, 100 g of solution contains 15 g of H2SO4
Then, mass H2SO4 in 110 g (100 cm3 ) of solution = 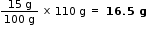
Mass of water in 110 g (100cm3 ) of solution = (110 - 16.5) g = 93.5 g
To obtain 100 cm3 of 15 % solution acid, we require the given information.Mass of water = 93.5 g
Mass of H2SO4 (100% pure) = 16.5 g
Since, the given sulphuric acid is 95% pure, hence,
Mass of H2SO4 (95%) will be required =
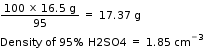
Answered by Hanisha Vyas | 06 Jul, 2015, 12:46: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 11:48: PM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 01:25: PM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by shamiyaali732 | 26 Sep, 2023, 02:00: AM
CBSE 12-science - Chemistry
Asked by elabarman58 | 23 Jan, 2023, 09:39: AM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 06:18: PM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 06:17: PM
CBSE 12-science - Chemistry
Asked by shiv.pama83 | 27 Nov, 2021, 05:50: AM
CBSE 12-science - Chemistry
Asked by mdarsadazizh | 31 Aug, 2021, 01:02: PM







