CBSE Class 12-science Answered
calculate the mass percentage of benzene and carbon tetrachloride. if 22g of benzene is dissolved in 122g of ccl4?
Asked by shiv.pama83 | 27 Nov, 2021, 05:50: AM
Mass of solution = Mass of benzene + Mass of carbon tetrachloride = 22 g + 122 g = 144 g
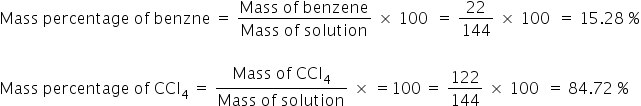
Answered by Ramandeep | 29 Nov, 2021, 18:22: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by soumyaranjanchhatria21 | 29 May, 2024, 08:20: AM
CBSE 12-science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 23:20: PM
CBSE 12-science - Chemistry
Asked by sameerteli003 | 08 Apr, 2024, 23:48: PM
CBSE 12-science - Chemistry
Asked by saritanohar22 | 13 Jan, 2024, 13:25: PM
CBSE 12-science - Chemistry
Asked by kamlesh.kumar.malee | 20 Dec, 2023, 06:59: AM
CBSE 12-science - Chemistry
Asked by shamiyaali732 | 26 Sep, 2023, 02:00: AM
CBSE 12-science - Chemistry
Asked by elabarman58 | 23 Jan, 2023, 09:39: AM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 18:18: PM
CBSE 12-science - Chemistry
Asked by gauravrastogi577 | 16 Aug, 2022, 18:17: PM
CBSE 12-science - Chemistry
Asked by shiv.pama83 | 27 Nov, 2021, 05:50: AM








