CBSE Class 9 Answered
what is the triple point of a substance
Asked by | 08 Jul, 2008, 08:08: PM
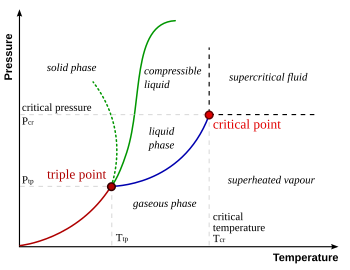
The triple point of a substance is the temperature and pressure at which three phases (gas, liquid, and solid) of that substance may coexist in thermodynamic equilibrium.
For example, the triple point temperature of mercury is at −38.8344 °C, at a pressure of 0.2 MPa.
The triple point of water is used to define the kelvin, the SI base unit of thermodynamic temperature. The number given for the temperature of the triple point of water is an exact definition rather than a measured quantity.
Answered by | 08 Jul, 2008, 10:00: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namratarnav123 | 21 Apr, 2024, 11:25: PM
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by sangeetha66599 | 25 Dec, 2022, 10:55: AM
CBSE 9 - Chemistry
Asked by pranavtamboli65.9 | 02 Aug, 2022, 08:12: PM
CBSE 9 - Chemistry
Asked by meghnahire17.9 | 17 Jul, 2022, 05:41: PM
CBSE 9 - Chemistry
Asked by rajuvrajuraju3 | 17 Jul, 2022, 11:05: AM
CBSE 9 - Chemistry
Asked by sanyaverma053 | 29 Jun, 2022, 10:55: AM