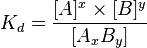CBSE Class 12-science Answered
what is the meaning of dissociation constant?
Asked by | 09 Oct, 2008, 10:28: AM
a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components,
The dissociation constant is usually denoted Kd and is the inverse of the affinity constant. In the special case of salts, the dissociation constant can also be called an ionization constant.
For a general reaction
in which a complex AxBy breaks down into x A subunits and y B subunits, the dissociation constant is defined
where [A], [B], and [AxBy] are the concentrations of A, B, and the complex AxBy, respectively.
Answered by | 10 Oct, 2008, 08:28: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by cjam41665 | 10 Oct, 2021, 12:56: AM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM