CBSE Class 9 Answered
What is mole
Asked by Shahbazalam1308 | 19 Feb, 2019, 01:49: PM
Mole concept-
1 mole of a substance is equal to its atomic mass or molecular mass expressed in grams.
The atomic mass of sodium is 23 grams.
Therefore, 23 grams of sodium is equal to one mole of sodium atoms.
Similarly, the molecular mass of oxygen (O2) = 2 × Atomic mass of oxygen
= 2 × 16 = 32 g
So, 32 grams of oxygen is equal to one mole of oxygen molecules.
1 mole (of anything) = 6.022 × 1023 in number
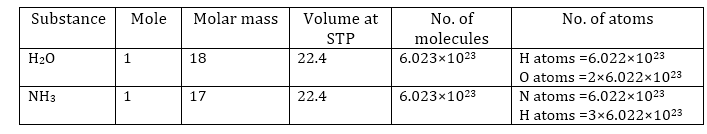
Answered by Varsha | 19 Feb, 2019, 02:24: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rajputanaji290 | 03 Oct, 2023, 09:30: PM
CBSE 9 - Chemistry
Asked by muditsharma287 | 09 Mar, 2023, 10:10: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:46: PM
CBSE 9 - Chemistry
Asked by shivalaxmi0205 | 08 Mar, 2023, 07:43: PM
CBSE 9 - Chemistry
Asked by jssjj | 19 Jan, 2023, 07:25: PM
CBSE 9 - Chemistry
Asked by mohammedhaqqani.6b | 14 Jun, 2022, 02:51: PM
CBSE 9 - Chemistry
Asked by gauravsingh36428 | 14 Mar, 2022, 07:44: PM
CBSE 9 - Chemistry
Asked by jiyajthakor | 28 Feb, 2022, 07:03: PM
CBSE 9 - Chemistry
Asked by gillsaabjashanpreetsingh3 | 16 Jan, 2022, 01:23: PM
CBSE 9 - Chemistry
Asked by prachisharma772007 | 16 Jan, 2022, 11:12: AM











