CBSE Class 10 Answered
what is Ionisation enthalpy?
Asked by | 08 Oct, 2008, 02:22: PM
The ionization enthalapy /ionization energy or EI of an atom or molecule is the energy required to remove an electron from the isolated atom or ion. More generally, the nth ionization energy is the energy required to strip it of the nth electron after the first n − 1 electrons have been removed. It is considered a measure of the "reluctance" of an atom or ion to surrender an electron, or the "strength" by which the electron is bound; the greater the ionization energy, the more difficult it is to remove an electron.
Answered by | 08 Oct, 2008, 03:13: PM
Application Videos
Concept Videos
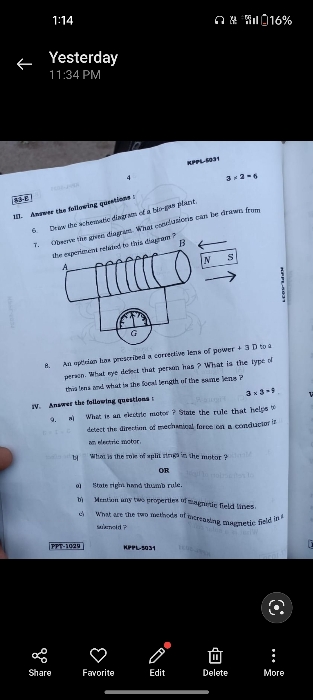
CBSE 10 - Physics
Asked by agankitgupta938 | 18 Apr, 2024, 04:29: PM
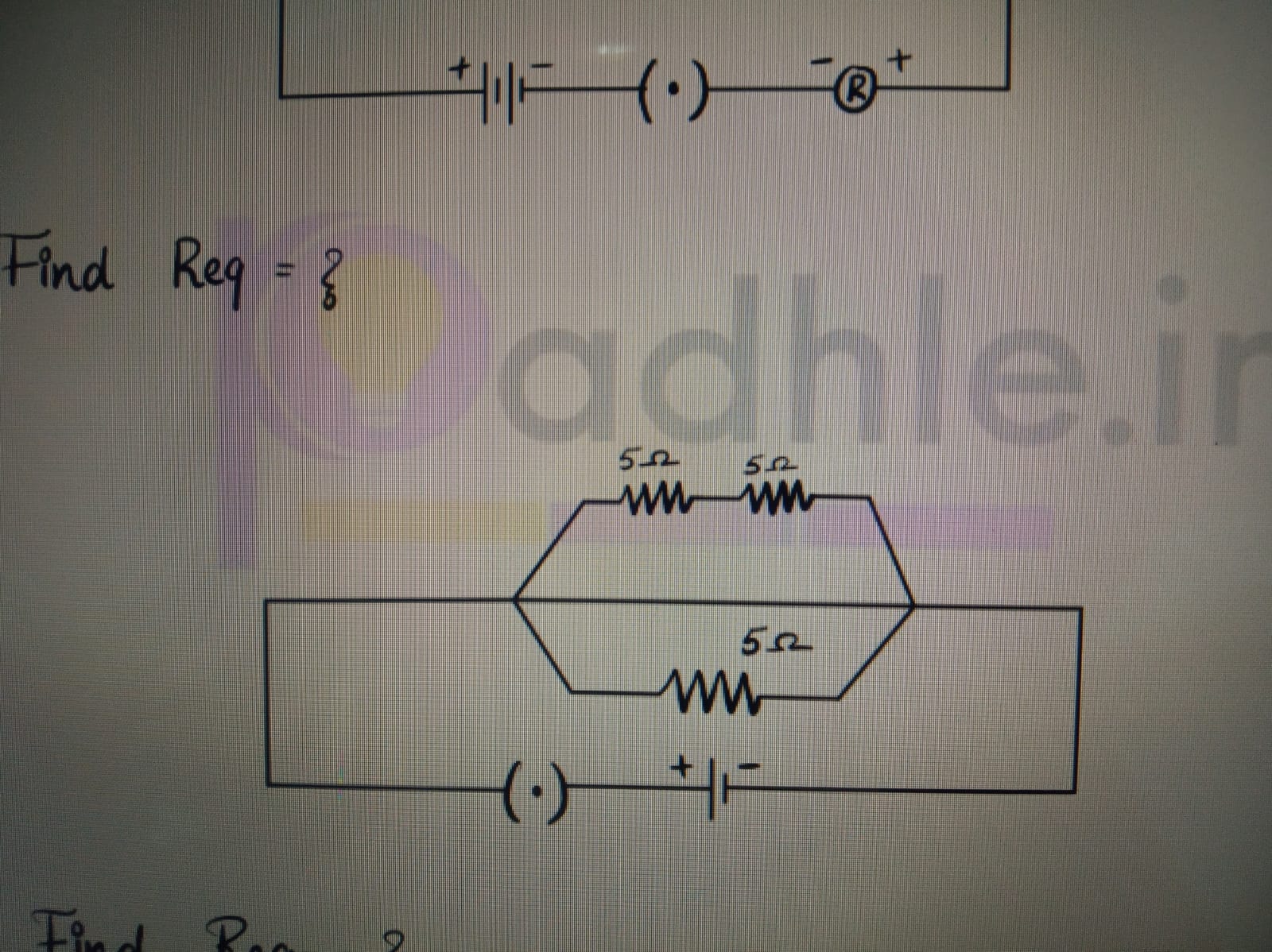
CBSE 10 - Physics
Asked by infinityupgraded | 13 Apr, 2024, 08:17: AM
CBSE 10 - Physics
Asked by suryamr2019 | 08 Mar, 2024, 04:32: PM
CBSE 10 - Physics
Asked by sheetal.kolte | 04 Mar, 2024, 12:38: PM
CBSE 10 - Physics
Asked by shrilakshmimunoli | 01 Mar, 2024, 01:15: AM
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by sailakshmi.avinesh | 13 Feb, 2024, 07:03: AM
CBSE 10 - Physics
Asked by saurabhjd527 | 30 Jan, 2024, 07:55: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM