CBSE Class 11-science Answered
What do you mean by phase change of matter?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
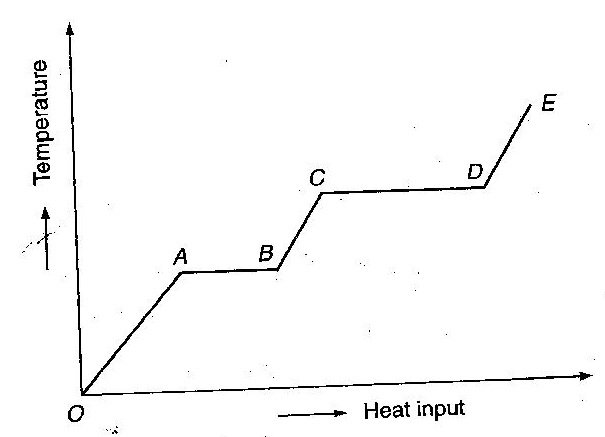
A phase change is a process accompanied by the emission or absorption of heat without any temperature change.
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 11-science - Physics
Asked by Aryan.suthar | 20 Apr, 2019, 21:34: PM
CBSE 11-science - Physics
Asked by Neeraj | 14 Apr, 2019, 22:41: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 15 Apr, 2015, 10:36: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 15 Apr, 2015, 10:39: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 15 Apr, 2015, 11:03: AM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM