CBSE Class 11-science Answered
What are fullerenes? Explain the structure of C60 molecule.
Asked by Topperlearning User | 31 May, 2016, 01:32: PM
- A new family of carbon allotropes consisting of cluster of carbon atoms such as C32 , C50 , C60 , C70 , C84 etc. are called fullerenes.
- Fullerenes are made by heating of graphite in an electric arc in the presence of inert gases such as helium or argon.
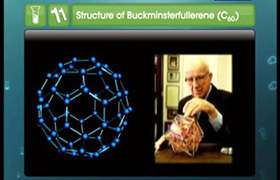
- Among these the allotrope having the molecular formula C60 is important. It is called Buckminster fullerene.
- C60 molecule is a perfect sphere and looks like a soccer ball and is also popularly known as bucky-ball.
- It contains twenty six- membered rings and twelve five membered rings.
- A six membered ring is fused with six or five membered rings but a five membered ring can only fuse with six membered rings.
- All the carbon atoms are equal and they undergo sp2 hybridisation.
- Each carbon atom forms three sigma bonds with other three carbon atoms.
- The remaining electron at each carbon is delocalised in molecular orbitals, which in turn give aromatic character to molecule.
- This ball shaped molecule has 60 vertices and each one is occupied by one carbon atom and it also contains both single and double bonds.
Answered by | 31 May, 2016, 03:32: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:42: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 11:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:32: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 11:46: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:37: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 12:50: PM