CBSE Class 11-science Answered
The volume occupied by a certain gas was found 5.6 dm3
when the pressure was 2 atmosphere. If the
pressure is increased by 15%, find the new volume of the gas.
Asked by shashank1854 | 28 May, 2019, 16:48: PM
In this question information is not given by its temperature.
So here i am assuming there is no change in temperature.
If Temperature is constant than apppying Boyle's law-
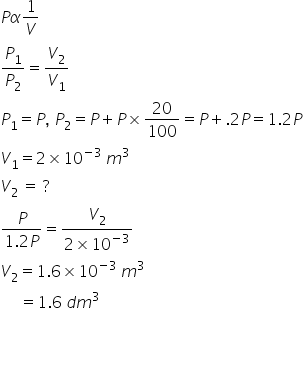
Answered by Ravi | 28 May, 2019, 18:28: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by mohd.arhaan812 | 06 May, 2024, 21:55: PM
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 22:43: PM
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 20:58: PM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 00:09: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 14:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 22:07: PM
CBSE 11-science - Chemistry
Asked by devanshuchhipani | 12 Mar, 2020, 20:42: PM
CBSE 11-science - Chemistry
Asked by ritua7330 | 01 Sep, 2019, 12:53: PM
CBSE 11-science - Chemistry
Asked by sajidivakaran | 21 Jul, 2019, 09:41: AM
CBSE 11-science - Chemistry
Asked by shashank1854 | 28 May, 2019, 16:48: PM