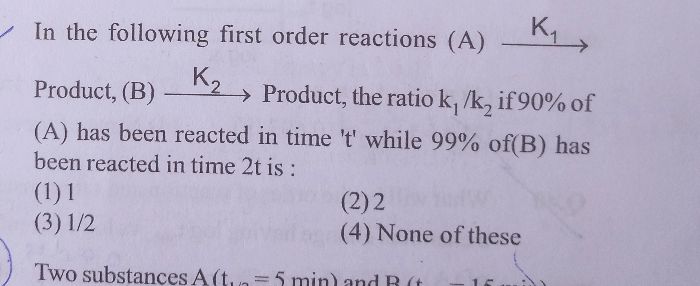CBSE Class 12-science Answered
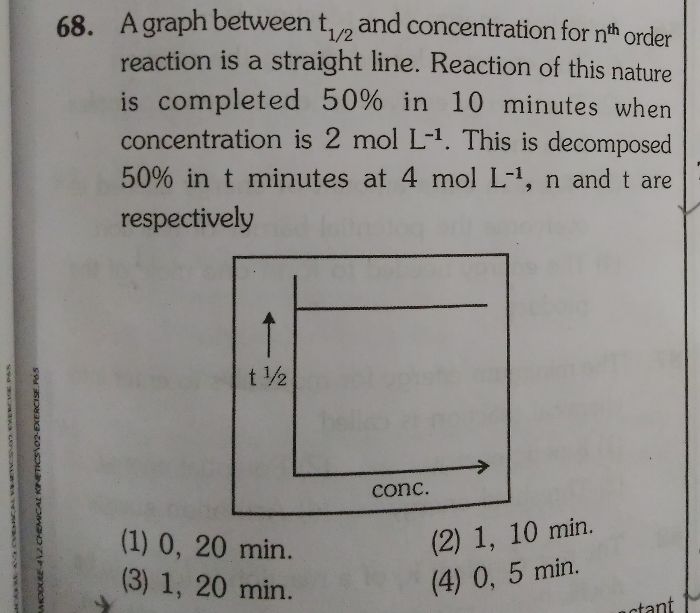
The integrated rate law for a zero-order reaction is [A]t-[A]0=kt derive an equation for the half-life of a zero-order reaction.
Asked by | 29 Nov, 2012, 11:47: AM
To illustrate this point, consider the reaction
A ? B
The rate of reaction, r, is given by
| r = - | d [A] d t |
Suppose this reaction obeys a first-order rate law:
r = k [A]
This rate law can also be written as
| r = - | d [A] d t |
= k [A] |
This equation is a differential equation that relates the rate of change in a concentration to the concentration itself. Integration of this equation produces the corresponding integrated rate law, which relates the concentration to time. When you viewed concentration-time curves in previous pages, you viewed the integrated rate laws.
| d [A] [A] |
= | - k d t |
At t = 0, the concentration of A is [A]0. The integrated rate law is thus
[A] = [A]0 e- k t
Answered by | 29 Nov, 2012, 03:58: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by cjam41665 | 10 Oct, 2021, 12:56: AM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM