CBSE Class 12-science Answered
the activation energy of a reaction is 75.2kj/mol in the absence of a catalyst and 50.14kj/mol with a catalyst.how many times will the rate of reaction grow in the presence of the catalyst if the reaction preceeds at 25degree celcius?
Asked by | 24 Jul, 2011, 04:02: AM
According to A rrhenius equation,

Let for uncatalysed reaction , 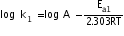 --------(i)
--------(i)
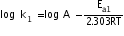 --------(i)
--------(i)And , for catalysed reaction ,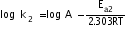 ---------(ii)
---------(ii)
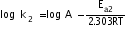 ---------(ii)
---------(ii)Subtracting equation (i) from equation (ii)
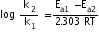
= 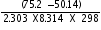
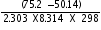
= 4.39
Taking antilog on both sides,we have
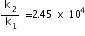
Therefore rate of reaction increased by 2.45 x 104 times
Answered by | 25 Jul, 2011, 10:01: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bhadauriyax | 30 Nov, 2023, 06:23: PM
CBSE 12-science - Chemistry
Asked by rahulbiswal946 | 08 Nov, 2023, 07:01: PM
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by cjam41665 | 10 Oct, 2021, 12:56: AM
CBSE 12-science - Chemistry
Asked by arshbhatia0809 | 22 Jul, 2021, 09:47: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 14 Jan, 2021, 12:40: PM
CBSE 12-science - Chemistry
Asked by Surendersingh0493 | 18 Oct, 2020, 02:05: PM
CBSE 12-science - Chemistry
Asked by khandarev3580 | 10 Oct, 2020, 10:54: AM
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by dr.akanksha0411 | 07 Aug, 2020, 11:56: AM










