CBSE Class 12-science Answered
State the type of mechanism in acid catalysed hydration of alkenes with reactions involved.
Asked by sd2021667 | 03 Dec, 2018, 09:12: AM
The addition of water to a double bond in presence of mineral acid according to Markonikov's rule is known as acid catalyzed hydration of an alkene.
The reaction takes place in three steps:
Step 1: Formation of carbocation :
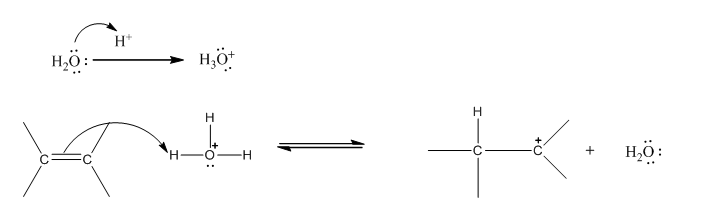
Step 2: Attack od lone pair of oxygen of water on carbocation:
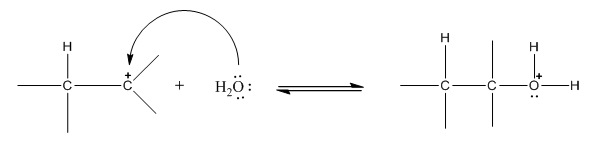
Step 3: Deprotonation to form alcohol:
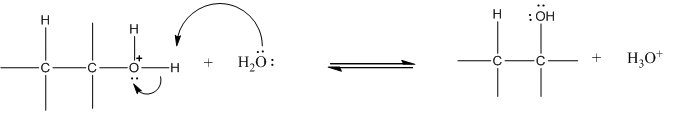
Answered by Ramandeep | 04 Dec, 2018, 11:34: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by bithikapurkait09052005 | 24 Jun, 2022, 08:35: AM
CBSE 12-science - Chemistry
Asked by sd2021667 | 03 Dec, 2018, 09:12: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Mar, 2014, 07:45: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 25 Mar, 2014, 17:08: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 12:46: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 12:54: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 12:56: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 12:58: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 13:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 08 Jun, 2016, 13:13: PM




