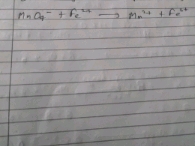CBSE Class 11-science Answered
Sir, how can we convert ppm into any other unit of mass?
Asked by | 07 Jun, 2008, 08:54: PM
ppm is equivalent to part per million by weight.i.e by level of contamination is 15 ppm we mean that if 1 kg of water s taken then content of CHCl3 is 15 × 10-6 kg.
Now no. of moles of CHCl3 = 15 × 10-3 /119.5=n(let)
then molality = n/(1-15 × 10-6)
Putting the value of n we get the molality of chloroform in solution.
Answered by | 18 Jun, 2008, 02:57: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM