CBSE Class 11-science Answered
Show four steps of carnot engine in P – V graph write the equation of each step and obtain the work
done by the system. Also obtain the efficiency of a carnot engine.
Asked by juzarsiddhapurwala | 07 Mar, 2021, 22:08: PM
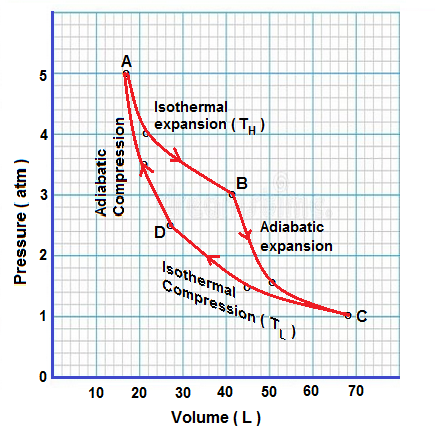
Carnot cycle consists of 4 process as explained below
(1) Isothermal expansion. Heat energy is absorbed from hot reservoir of high temperature TH ans work is done by system.
In figure, this process is shown from state A to state B
(2) Adiabatic expansion . System is cooled from High temperature TH to lower temperature TL adiabatically.
In figure, this process is shown from state B to state C
(3) Isothermal compression . Thermodynamic fuel is compressed to intermediate pressure isothermally at low temperature TL.
In figure, this process is shown from state C to state D
(4) adiabatic compression . Thermodynamic fuel is compressed adiabatically to initial pressure to close the cycle.
In figure , this process is shown from state D to state A
| Process | Heat Transfer Q | Workdone W | Remarks |
|
state A to state B
Isothermal expansion
|
QAB = n R TH ln( VB / VA ) | WAB = n R TH ln( VB / VA ) |
From First law of thermodynamics , Q = ΔU+W
Where Q is heat trasferred , ΔU is change in internal energy
and W is workdone . For isothermal process ΔU = 0
Hence we have Q = W
n is number of moles , R is universal gas constant,
TH is hot reservoir temperature,
VA and VB are volume at state-A and state-B respectively
|
|
State B to State C
Adiabatic expansion
|
QBC = 0 | WBC = [ n R ( TL - TH) ] / (1 - γ) |
Adiabatic process heat transfer is zero
TL is cold reservoir temperature and
γ is ratio of specific heat of thermodynamic fuel
|
|
State C to state D
Isothermal Compression
|
QCD = n R TL ln( VD / VC ) | WCD = n R TL ln( VD / VC ) |
VC and VD are volume at state-C and state-D respectively
|
|
State D to State A
Adiabatic compression
|
QDA = 0 | WDA = [ n R ( TH - TL) ] / (1 - γ) |
Net Workdone = WAB + WCD = n R ( TH - TL ) ln( VB / VA )
( In Carnot cycle , VB / VA = ( VC / VD ) , i.e. volume ratio for both isothermal process is same ,
We have used this in above expression to get net heat transfer )
Heat transfer from Hot reservoir = QAB = n R ( TH - TL ) ln( VB / VA )
Efficiency = Net workdone / Heat transfer from Hot reservoir
Efficiency = [ n R ( TH - TL ) ln( VB / VA ) ] / [ n R TH ln( VB / VA )]
Efficiency = ( TH - TL ) / TH = [ 1 - ( TL / TH ) ]
Answered by Thiyagarajan K | 08 Mar, 2021, 00:33: AM
Concept Videos
CBSE 11-science - Physics
Asked by juzarsiddhapurwala | 07 Mar, 2021, 22:08: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 13:47: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 17 Apr, 2015, 13:47: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM