ICSE Class 10 Answered
Q. 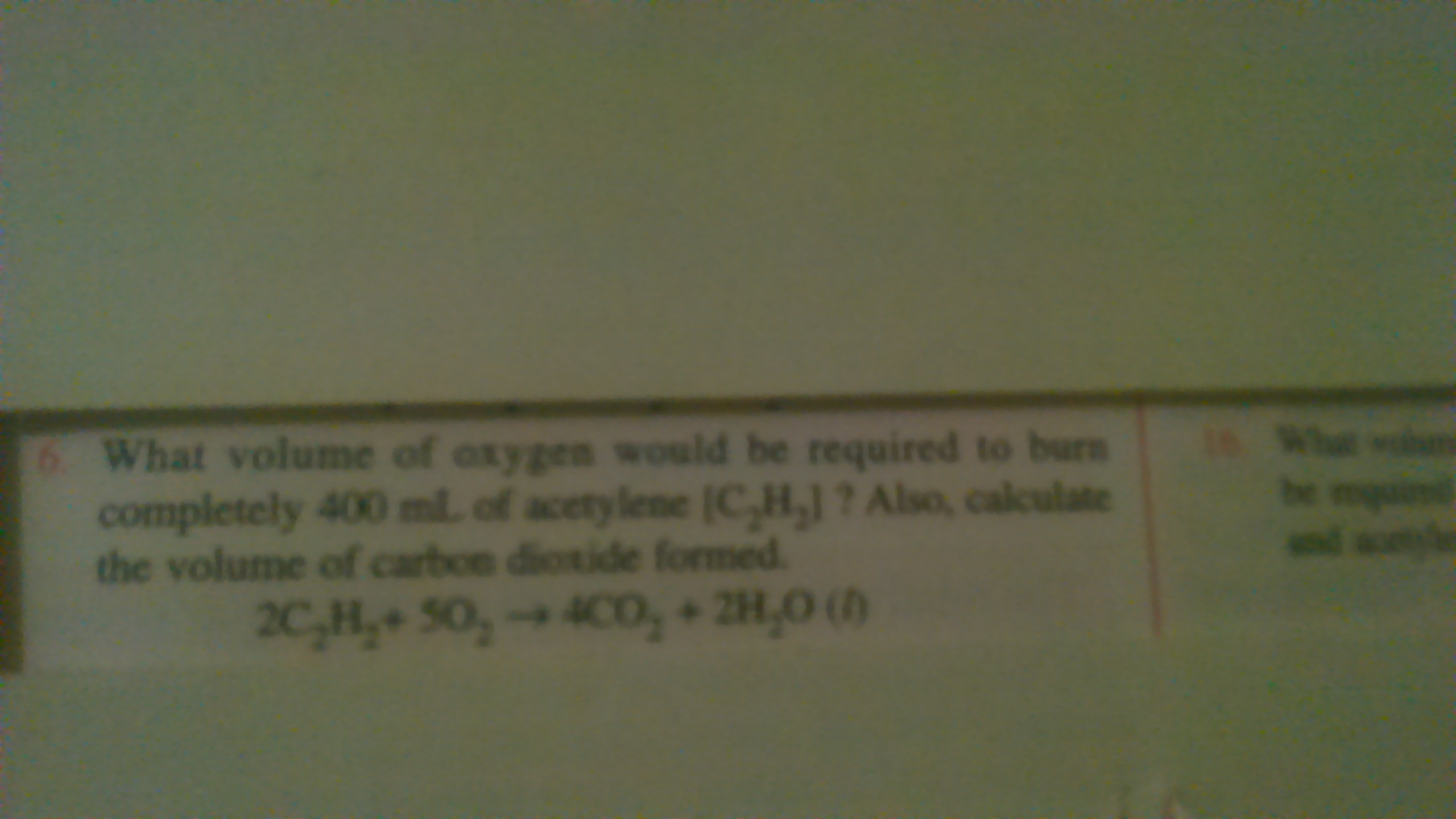
Asked by Invest123736 | 23 May, 2016, 09:58: PM
2C2H2 + 5O2 → 4CO2 + 2H2O (l)
2 V 5 V 4 V
From equation, 2 V of C2H2 requires = 5 V of O2
So, for 400ml C2H2 , O2 required = 400 X 5/2 =1000 ml
Similarly, 2 V of C2H2 gives = 4 V of CO2
So, 400ml of C2H2 gives CO2 = 400 x 4/2 = 800ml
Answered by Vaibhav Chavan | 24 May, 2016, 10:49: AM
Concept Videos
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 05 Mar, 2024, 06:40: PM
ICSE 10 - Chemistry
Asked by kundus458 | 07 Feb, 2024, 08:55: AM
ICSE 10 - Chemistry
Asked by matloobser | 07 Sep, 2023, 09:36: AM
ICSE 10 - Chemistry
Asked by dafk04.dp | 06 May, 2021, 06:22: PM
ICSE 10 - Chemistry
Asked by amit.clw4 | 15 Mar, 2021, 07:27: AM
ICSE 10 - Chemistry
Asked by amit.clw4 | 14 Mar, 2021, 08:12: AM
ICSE 10 - Chemistry
Asked by ravi.solaabhi | 17 Oct, 2020, 10:11: AM
ICSE 10 - Chemistry
Asked by payalagrawal1724 | 28 Jun, 2020, 07:22: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 29 Dec, 2019, 08:07: PM
ICSE 10 - Chemistry
Asked by Dsangayy | 08 May, 2019, 07:11: PM






