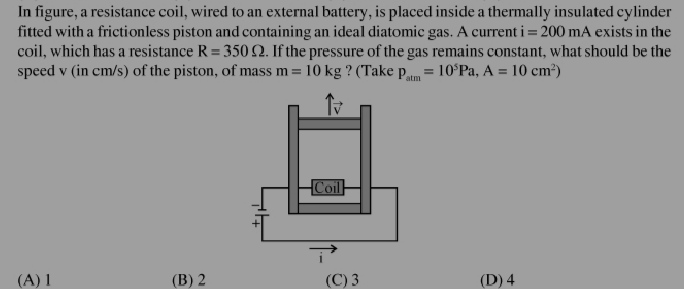
JEE Class main Answered
plz solve my doubt

Asked by manvirsingh2242 | 11 Jun, 2022, 09:14: AM
when two gases at same pressure and same temperature are allowed to mix ,
then final temperature will remain same as initial temperature.
hence this mixing process is isothermal expansion process for both the gases.
Change in entropy of isothermal expansion process, ΔS = n R ln( Vf / Vi )
where n is number of moles , R is universal gas constant , Vi is initial volume and Vf is final volume.
If N is number of molecules , n mole = N / NA , where NA is avagadro number .
Change in entropy, ΔS = N ( R / NA ) ln( Vf / Vi ) = N k ln( Vf / Vi )............................ (1)
where ( R / NA ) = k is Boltzman's constant
Vf = final volume = V1 + V2 , where V1 is initial volume occupied by N1 molecules of gas-1 and
V2 is initial volume occupied by N2 molecules of gas-2 .
Since at same temperature and pressure , volume is proportional to number of moles or number of molecules
we write eqn.(1) for entropy change of mixing of both the gases as
ΔS = N1 k ln[ ( N1 + N2 ) / N2 ) + N2 k ln[ ( N1 + N2 ) / N2 )
if N1 ≈ N2 , we get , ΔS = ( N1 + N2 ) k ln(2)
Answered by Thiyagarajan K | 11 Jun, 2022, 16:43: PM
JEE main - Physics
Asked by rambabunaidu4455 | 03 Oct, 2024, 16:03: PM
JEE main - Physics
Asked by yashu22022006 | 25 May, 2024, 09:13: AM
JEE main - Physics
Asked by pataiyalalit02 | 19 May, 2024, 16:53: PM
JEE main - Physics
Asked by hridayjayaram085 | 12 Jan, 2024, 17:50: PM
JEE main - Physics
Asked by ashainy91829 | 06 Nov, 2023, 13:08: PM
JEE main - Physics
Asked by ghrushi3 | 02 Nov, 2023, 22:05: PM
JEE main - Physics
Asked by samarthghogare | 06 May, 2023, 11:17: AM
JEE main - Physics
Asked by aadityakumar0603 | 06 Mar, 2023, 22:03: PM
JEE main - Physics
Asked by manvirsingh2242 | 21 Jun, 2022, 16:35: PM