JEE Class main Answered
pls solve

Asked by walavalkarsandeep44 | 22 Jun, 2022, 19:54: PM
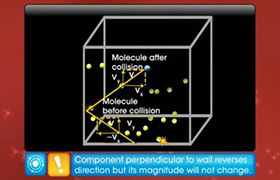
Change in momenum Δp for one H2 molecule after collision, Δp = ( 2 m v cos45)
Force exerted on wall = rate of change of momentum = Δp / Δt = ( 2 m v cos45)
( Δt = 1 sec )
Force exerted on wall due to n molecules after collision = ( 2 n m v cos45)
Pressure = Force / Area = ( 2 n m v cos45) / A ......................... (1)
Number of H2 molecule hitting the wall = n = 1023
mass of H2 molecule = m = 3.22 × 10-27 kg
speed of each molecule = v = 103 m/s
Area = A = 2 cm2 = 2 × 10-4 m2
Pressure is determined from eqn.(1) as
P = ( 2 × 1023 × 3.22 × 10-27 × 103 × cos45 ) / ( 2 × 10-4 ) Pa
P = 4554 Pa
Answered by Thiyagarajan K | 22 Jun, 2022, 21:46: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by ojasgarg96 | 26 Feb, 2023, 22:06: PM
JEE main - Physics
Asked by aaryamanmodern | 17 Jan, 2023, 22:18: PM
JEE main - Physics
Asked by soumendra.mohanty42 | 31 Dec, 2022, 18:49: PM
JEE main - Physics
Asked by walavalkarsandeep44 | 22 Jun, 2022, 19:54: PM
JEE main - Physics
Asked by chackopappan48 | 23 May, 2020, 14:20: PM