JEE Class main Answered
KTG

Asked by nikhilvats208 | 07 Feb, 2024, 09:16: PM
RMS speed v of hydrogen molecules at temperature T is
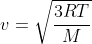
where M is molar mass and R is universal gas constant .
if m is mass of one molecule of hydrogen gas and n is number of gas molecules in the container ,
then total momentum P is
P = n × m × v
if quantity of hydrogen gas in container is 1 mole , then product of n and m is one molar mass M
Hence total momentum is
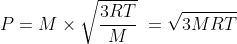
Let us substitute molar mass M = 2.0169 × 10-3 kg , R = 8.314 J/(K mole) and T = 300 K, we get
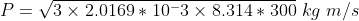
P = 3.885 kg m/s
Answered by Thiyagarajan K | 08 Feb, 2024, 01:48: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by ojasgarg96 | 26 Feb, 2023, 10:06: PM
JEE main - Physics
Asked by aaryamanmodern | 17 Jan, 2023, 10:18: PM
JEE main - Physics
Asked by soumendra.mohanty42 | 31 Dec, 2022, 06:49: PM
JEE main - Physics
Asked by walavalkarsandeep44 | 22 Jun, 2022, 07:54: PM
JEE main - Physics
Asked by chackopappan48 | 23 May, 2020, 02:20: PM