CBSE Class 12-science Answered
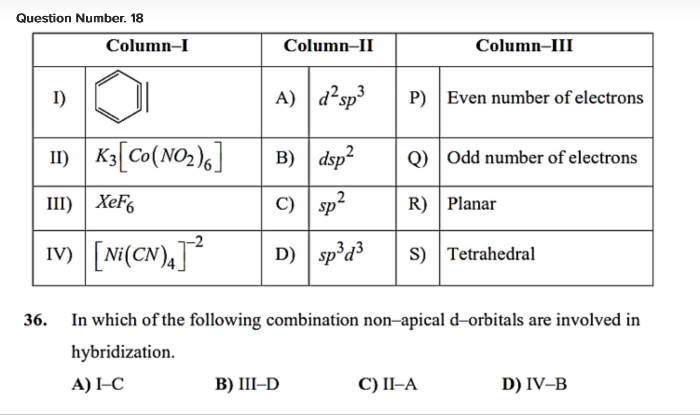
Please answer Q.17 from board paper.
Assertion (A): Nucleophilic substitution of iodoethane is easier than chloroethane.
Reason (R): Bond energy of C — CI bond is less than C — I bond.
Asked by anubhutiupadhaya | 01 Mar, 2023, 12:30: PM
Dear Student,
Correct option - (a) A and R are true and R is the correct explanation of A.
Solution:
In Iodoethane, the bonding is between s orbital and 3 P orbitals, while in case of chloroethane, the bonding is between 2p and 3p orbital which is more effective.
Also, since less bond energy, it is easier to break C-I bond and hence, easier nucleophilic substitution.
Answered by | 01 Mar, 2023, 17:28: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by vvimla042 | 09 Jul, 2024, 19:48: PM
CBSE 12-science - Chemistry
Asked by ankitmonda.bankhatil | 11 Jun, 2024, 19:10: PM
CBSE 12-science - Chemistry
Asked by soumeshmishra08 | 07 Jun, 2024, 08:21: AM
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by soumyaranjanchhatria21 | 29 May, 2024, 08:20: AM
CBSE 12-science - Chemistry
Asked by adarshsingh | 23 May, 2024, 23:14: PM
CBSE 12-science - Chemistry
Asked by gupta.sandhya2007 | 23 May, 2024, 08:16: AM
CBSE 12-science - Chemistry
Asked by desaianant541 | 15 May, 2024, 21:05: PM
CBSE 12-science - Chemistry
Asked by anithaanu629940 | 11 May, 2024, 12:31: PM