CBSE Class 12-science Answered
Please answer fast.
Write the equations involved in the presentation of KMnO4 from Pyrolusite ore

Asked by varma.renu9481 | 01 Mar, 2023, 12:09: PM
Dear Student,
The correct question is:
Write the equations involved in the preparation of KMnO4 from Pyrolusite ore.
It is prepared from the mineral, pyrolusite, MnO2, for large scale preparation.
The preparation involves following steps;
(a) Conversion of MnO2 into potassium manganate:
The finely crushed pyrolusite mineral is fused with potassium hydroxide in presence of air or oxidizing agent such as potassium nitrate. The green coloured potassium manganate is formed.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
2MnO2 + 2K2CO3 + O2 → 2K2MnO4 + 2CO2
MnO2 + 2KOH + KNO3 → K2MnO4 + KNO2 + H2O
3MnO2 + 6KOH + KClO3 → 3K2MnO4 + KCl + 3H2O
If this solution is allowed to stand for some time, potassium manganate is then undergoes disproportionation in the neutral or acidic solution.
Answered by | 01 Mar, 2023, 13:00: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by vvimla042 | 09 Jul, 2024, 19:48: PM
CBSE 12-science - Chemistry
Asked by ankitmonda.bankhatil | 11 Jun, 2024, 19:10: PM
CBSE 12-science - Chemistry
Asked by soumeshmishra08 | 07 Jun, 2024, 08:21: AM
CBSE 12-science - Chemistry
Asked by routraypriyanka255 | 04 Jun, 2024, 23:43: PM
CBSE 12-science - Chemistry
Asked by BABUYVU | 02 Jun, 2024, 09:30: AM
CBSE 12-science - Chemistry
Asked by soumyaranjanchhatria21 | 29 May, 2024, 08:20: AM
CBSE 12-science - Chemistry
Asked by adarshsingh | 23 May, 2024, 23:14: PM
CBSE 12-science - Chemistry
Asked by gupta.sandhya2007 | 23 May, 2024, 08:16: AM
CBSE 12-science - Chemistry
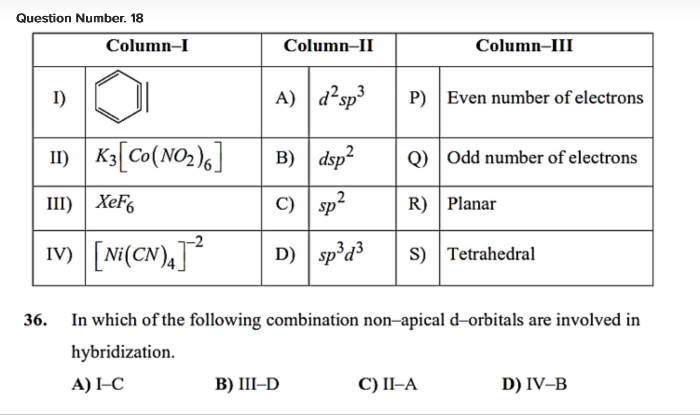
Asked by desaianant541 | 15 May, 2024, 21:05: PM
CBSE 12-science - Chemistry
Asked by anithaanu629940 | 11 May, 2024, 12:31: PM