CBSE Class 11-science Answered
mass of aluminium is 500 gm .how much amount of heat needs to supply in order to increase its temperature by 40 degree celcius
Asked by Leenatripathi20 | 10 Feb, 2020, 19:12: PM
Given:
Mass of Al m = 500 gm
ΔT = 40 °C
We knw,
Specific heat capacity of Al = 0.9 J/g°C
We have,
Heat reaquired, Q = m × c × ΔT
= 500 × 0.9 × 40
= 18000 J
= 18 kJ
Answered by Varsha | 11 Feb, 2020, 13:02: PM
CBSE 11-science - Chemistry
Asked by rukayabatool395 | 24 Mar, 2024, 19:48: PM
CBSE 11-science - Chemistry
Asked by rama26516 | 12 Mar, 2022, 13:49: PM
CBSE 11-science - Chemistry
Asked by mossewalasindhu | 20 Nov, 2021, 14:16: PM
CBSE 11-science - Chemistry
Asked by chandankumargochhayat6 | 19 May, 2021, 08:48: AM
CBSE 11-science - Chemistry
Asked by saimahima2005 | 16 Jul, 2020, 18:28: PM
CBSE 11-science - Chemistry
Asked by Leenatripathi20 | 10 Feb, 2020, 19:12: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 26 Aug, 2019, 23:41: PM
CBSE 11-science - Chemistry
Asked by hritikch37 | 16 Dec, 2018, 22:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 14:53: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 11:18: AM

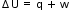 .
.