ICSE Class 10 Answered
Is H3PO3 a dibasic acid or a tribasic acid ?
Asked by anukesh.j | 07 Nov, 2015, 05:37: PM
H3PO3 has three hydrogen atoms and looking at the structure it is clear that in H3PO3 only two hydrogen atoms are joined through the oxygen atoms and are ionisable. The third hydrogen atom is linked to P-atom directly and is not ionisable. The H-atom joined to the P- atom is reducing in nature and is not ionisable as a proton and therefore H3PO3 acts as a dibasic acid.
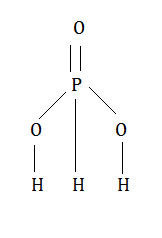
Answered by Vaibhav Chavan | 30 Nov, 2015, 12:40: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by artee.misra | 01 Jun, 2021, 05:54: PM
ICSE 10 - Chemistry
Asked by surendarkaur288 | 22 Apr, 2021, 03:56: PM
ICSE 10 - Chemistry
Asked by saibaba1069 | 24 Mar, 2020, 11:13: AM
ICSE 10 - Chemistry
Asked by pb_ckt | 04 Feb, 2019, 12:24: PM
ICSE 10 - Chemistry
Asked by malapandey8278 | 01 Feb, 2019, 05:53: AM





