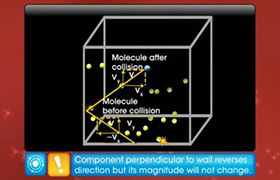
JEE Class main Answered
In this quesn, how they have assumed thta final pressure of both the vessels will be same.
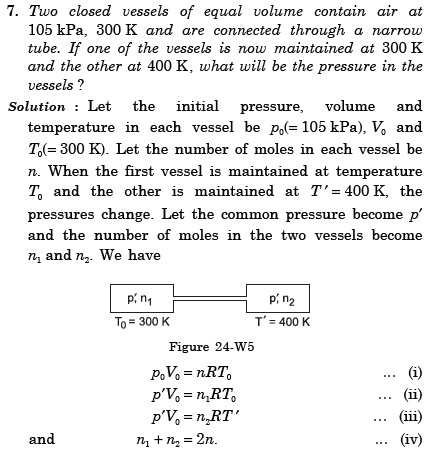
Asked by aaryamanmodern | 17 Jan, 2023, 22:18: PM
Initially both vessels of equal volume V is at same pressure Po and absolute temperature To .
Let n be number of moles in each vessel . Hence we write ideal gas equation as
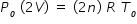
where R is universal gas constant. Hence we write
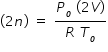 ..............................................(1)
..............................................(1)If one vessel is maintained at temperature To and other one is maintained at temperature T1 ( T1 > To ) ,
then vessel at temperature To will have more number of moles and vessel at temperature T1
will have less number of moles because both vessels are connected by a narrow tube
and they are at equal pressure.
Let n1 be the number of moles in the vessel that is maintained at temperature To .
Let n2 be the number of moles in the vessel that is maintained at temperature T1 .
Then we have , 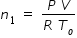 .............................(2)
.............................(2)
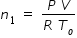 .............................(2)
.............................(2)and 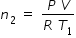 ......................................(3)
......................................(3)
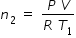 ......................................(3)
......................................(3)By adding eqn.(2) and (3) , we get
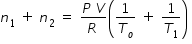 .............................(4)
.............................(4) Since number of moles in both vessels remain constant, n1 + n2 = ( 2 n )
Hence we equate eqn.(1) and (4) to get
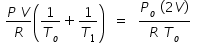
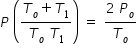
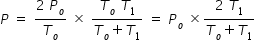
Let us substitute values , Po = 105 kPa , To = 300 K and T1 = 400 K
P = 105 × ( 800/700) = 120 kPa
Answered by Thiyagarajan K | 17 Jan, 2023, 23:40: PM
Application Videos
Concept Videos
JEE main - Physics
Asked by ojasgarg96 | 26 Feb, 2023, 22:06: PM
JEE main - Physics
Asked by aaryamanmodern | 17 Jan, 2023, 22:18: PM
JEE main - Physics
Asked by soumendra.mohanty42 | 31 Dec, 2022, 18:49: PM
JEE main - Physics
Asked by walavalkarsandeep44 | 22 Jun, 2022, 19:54: PM
JEE main - Physics
Asked by chackopappan48 | 23 May, 2020, 14:20: PM