CBSE Class 10 Answered
In the following questions (13-16), a statement of assertion followed by a statement of
reason is given. Choose the correct answer out of the following choices. a) Assertion
and reason both are correct statements and reason is correct explanation for
assertion. b) Assertion and reason both are correct statements but reason is not
correct explanation for assertion. c) Assertion is correct statement but reason is wrong
statement. d) Assertion is wrong statement but reason is correct statement.
a. Assertion: When zinc granules are added to dilute hydrochloric acid effervescence
occurs.
Reason: Formation of CO2 gas is the cause of effervescence.
Asked by palakkothari46 | 22 Jun, 2021, 11:58: AM
When hydrochloric acid reacts with zinc than it forms Zinc chloride and releases hydrogen gas.
Chemical equation for this-
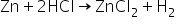
This efferevescence is caused by hydrogen gas. It is not caused by CO2 .
So, Correct option is (c). Assertion is correct statement but reason is wrong.
Answered by Ravi | 23 Jun, 2021, 11:06: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by aggrwalmontek | 14 Sep, 2023, 22:43: PM
CBSE 10 - Chemistry
Asked by manisha.5154 | 15 Jun, 2022, 14:52: PM
CBSE 10 - Chemistry
Asked by ranishoba947 | 10 May, 2022, 21:04: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 00:29: AM
CBSE 10 - Chemistry
Asked by anshika.dubey9809 | 11 Nov, 2021, 19:42: PM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 10 Jul, 2021, 22:27: PM
CBSE 10 - Chemistry
Asked by palakkothari46 | 22 Jun, 2021, 11:58: AM
CBSE 10 - Chemistry
Asked by nitikakaliramana466 | 14 May, 2021, 09:37: AM
CBSE 10 - Chemistry
Asked by ayan1.chatterjee | 07 May, 2021, 20:05: PM










