CBSE Class 12-science Answered
If photon is a particle then why do we associate wavelength and frequency with it?
Asked by Nishtha Sardana | 23 Nov, 2014, 09:08: PM
Light radiation is made up of tiny packets of energy called quanta. One quantum of light radiation is called photon which travels with speed of light. Radiation has dual nature, it possessses properties of both wave and particle. Moving material particle acts as wave or particle. The wave associated with moving material particle is called matter wave which has wavelength also called de-Broglie wavelength is given as,
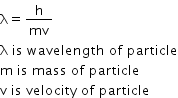
Answered by Priyanka Kumbhar | 24 Nov, 2014, 10:30: AM
Concept Videos
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 07:17: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM