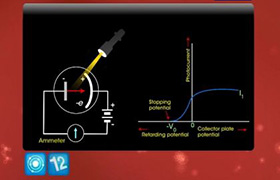
CBSE Class 12-science Answered
Why are alkali metals most suited for photoelectric emission?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
The work function of alkali metals is quite low (e.g. for potassium, it is 2.3 eV and for sodium is 2 eV). As a result, they show photoelectric effect even with visible light. For this reason, alkali metals are most suited for photoelectric emission.
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 12-science - Physics
Asked by basithhhabduuu | 14 Jul, 2024, 17:07: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 18:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 19:17: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM