CBSE Class 10 Answered
i havea doubt in this equation which is likethis given below -
i) Iron react with steam to form the metal oxide and hydrogen.
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
(1) why the there is Fe3O4 in the above equation and why not Fe2O3
(2)kindely explain me how we came to this equation
(2)(a) What is the equation for the reaction of calcium and potassium in water?
Calcium and potassium do not react with each other, but each decomposes water to make the relevant hydroxide with the release of hydrogen. Ca + 2H₂O → Ca(OH)₂ + H₂. 2K + 2H₂O → 2KOH + H₂.
Asked by aayushsoni7474 | 25 Apr, 2020, 12:26: PM
(1)
When iron reacts with steam, it forms metal oxides and hydrogen gas.
Iron reacts to form iron(II,III) oxide [FeO+Fe2 O3 ] and H2 gas.
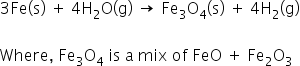
(2)
Sodium, potassium and calcium reacts with cold water to form hydroxide with the liberation of hydrogen gas.
2K + 2H2O → 2KOH + H2
2Na + 2H2O → 2NaOH + H2
Ca + 2H2O → Ca(OH)2 + H2
Answered by Ramandeep | 27 Apr, 2020, 11:56: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by ritik9897022 | 05 Feb, 2024, 09:42: PM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 05 Oct, 2021, 09:18: AM
CBSE 10 - Chemistry
Asked by virkarman36 | 08 Aug, 2021, 09:24: AM
CBSE 10 - Chemistry
Asked by dnupadhyay214 | 13 Mar, 2021, 12:01: PM
CBSE 10 - Chemistry
Asked by Vishavjet567 | 31 Oct, 2020, 10:52: AM
CBSE 10 - Chemistry
Asked by aryanluniwal1516 | 12 Sep, 2020, 11:43: AM
CBSE 10 - Chemistry
Asked by broprint18 | 07 Jun, 2020, 04:16: PM
CBSE 10 - Chemistry
Asked by prakharsingh167 | 25 May, 2020, 10:20: PM
CBSE 10 - Chemistry
Asked by sonaliagarwal172 | 16 May, 2020, 10:16: AM