CBSE Class 10 Answered
ans
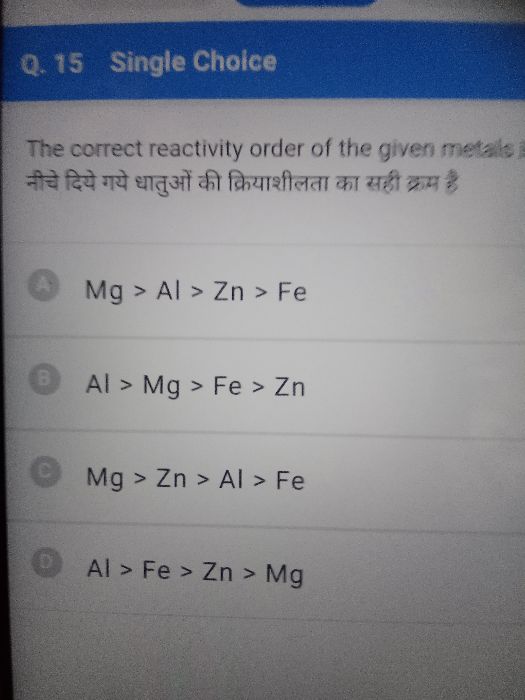
Asked by raj99mak | 19 Jul, 2020, 11:27: AM
The arrangement of metals in a vertical column in the order of decreasing reactivities is called reactivity series of metals.
The most reactive metals are placed at the top and least reactive metals are placed at the bottom of the reactivity series.
The reactivity series is:
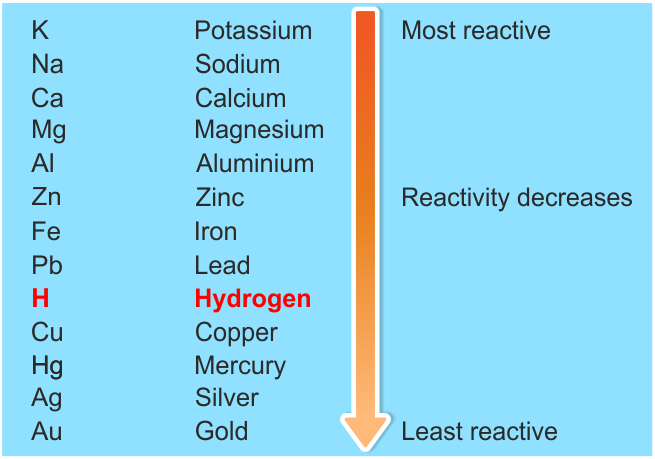
So, Order will be- Mg>Al>Zn>Fe
Answered by Ravi | 20 Jul, 2020, 19:02: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by ritik9897022 | 05 Feb, 2024, 21:42: PM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 05 Oct, 2021, 09:18: AM
CBSE 10 - Chemistry
Asked by virkarman36 | 08 Aug, 2021, 09:24: AM
CBSE 10 - Chemistry
Asked by dnupadhyay214 | 13 Mar, 2021, 12:01: PM
CBSE 10 - Chemistry
Asked by Vishavjet567 | 31 Oct, 2020, 10:52: AM
CBSE 10 - Chemistry
Asked by aryanluniwal1516 | 12 Sep, 2020, 11:43: AM
CBSE 10 - Chemistry
Asked by broprint18 | 07 Jun, 2020, 16:16: PM
CBSE 10 - Chemistry
Asked by prakharsingh167 | 25 May, 2020, 22:20: PM
CBSE 10 - Chemistry
Asked by sonaliagarwal172 | 16 May, 2020, 10:16: AM