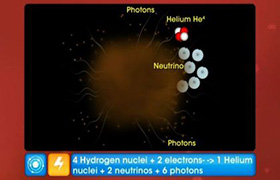
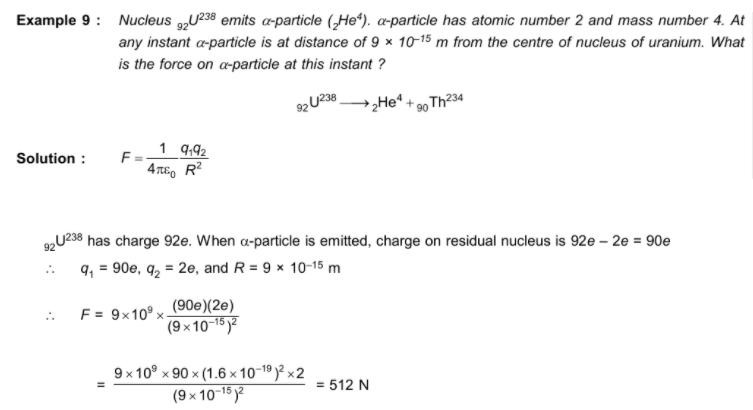
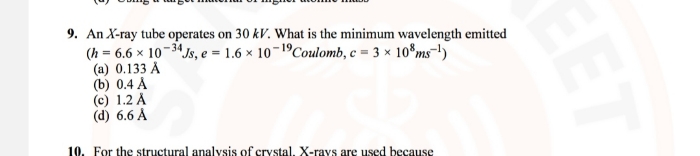NEET Class neet Answered
how to solve this ques?

Asked by begfatima123 | 06 May, 2022, 23:37: PM
Energy of electron orbits in hydrogen atom is given as
En = -13.6 / n2 ........................... (1)
Where n is the orbital quantum number . For ground state n =1 , for first excited state n =2 so on
The negative sign of the total energy of an electron moving in an orbit means that
The negative sign of the total energy of an electron moving in an orbit means that
the electron is bound with the nucleus. Energy will thus be required to remove the electron from
the hydrogen atom to a distance infinitely far away from its nucleus .
At room temperature, most of the hydrogen atoms are in ground state.
When a hydrogen atom receives energy by incident photon, the atom may acquire sufficient energy
to raise the electron to higher energy states. The atom is then said to be in an excited state.
From Eq. (1), for n = 3; the energy E3 is –1.51 eV and E3 – E1 = 12.09 eV,
or to excite the hydrogen atom from its ground state (n = 1) to second excited state (n = 3),
12.09 eV energy is required,
Hence the incident 12.1 eV photon energy raises the ground state electron to second excited state.
If transition is made from second excited state ( n = 3) to the ground state ( n =1 ),
we get a line spectrum that belongs to Lyman series
Answered by Thiyagarajan K | 07 May, 2022, 08:09: AM
Concept Videos
NEET neet - Physics
Asked by roshanrocky334 | 13 Jan, 2024, 11:52: AM
NEET neet - Physics
Asked by adititiwari601 | 13 Jun, 2022, 07:44: AM
NEET neet - Physics
Asked by begfatima123 | 06 May, 2022, 23:37: PM
NEET neet - Physics
Asked by jhajuhi19 | 30 Aug, 2021, 20:02: PM
NEET neet - Physics
Asked by akshadevdm2020 | 22 May, 2021, 15:43: PM
NEET neet - Physics
Asked by akdwadasi1111 | 20 Apr, 2021, 12:19: PM
NEET neet - Physics
Asked by Prashant DIGHE | 10 Apr, 2020, 21:21: PM