CBSE Class 12-science Answered
how that de Broglie hypothesis of matter waves is in agreement with Bohr's theory.
Asked by mahesh010167 | 03 Mar, 2015, 08:26: PM
The de-Broglie hypothesis of matter wave supports the Bohr's concept of stationary orbit.
According to de-Broglie hypothesis, a wave is associated with moving material particle which controls the particle in all aspects.This wave associated with moving particle is called matter wave whose wavelength is called de-Broglie wavelength.The wavelength λ of the wave associated with electron while moving with velocity v is given by:
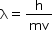 ---------(1)
---------(1)where h is the planck's constant and m the mass of the particle.
According to this hypothesis, a stationary orbit is that orbit whose circumference is integral mutiple of wavelength of wave associated with electron in that orbit.
Let λ be the de-Broglie wavelength of electron while revolving in nth orbit of radius r, then we can write it as:

This is as stated by Bohr's postulate about stable orbits.
i.e. Electrons revolve only in stationary orbits for which total angular momentum of the revoving electron is an integral mutiple of h/2π
i.e. mvr =n(h/2π)
Answered by Jyothi Nair | 04 Mar, 2015, 01:07: PM
Concept Videos
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by shivakumarshreyas24 | 01 Mar, 2020, 08:12: AM
CBSE 12-science - Physics
Asked by khushimassey437 | 31 May, 2019, 08:41: AM
CBSE 12-science - Physics
Asked by manasvijha | 19 Mar, 2019, 07:17: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM