ICSE Class 10 Answered
how nitrogen molecule is form
Asked by vijayvijay09644 | 06 Mar, 2024, 22:37: PM
Dear Student,
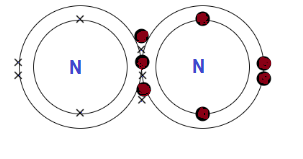
The atomic number of nitrogen is 7.
So, it has 7 electrons and the outermost electronic configuration is 1s2 2s2 2p3.
Three unpaired electrons present in the p orbital would form triple bonding with another nitrogen atom by sharing electrons.
There would be one covalent bond and two pi bonds.
Answered by | 07 Mar, 2024, 10:14: AM
Concept Videos
ICSE 10 - Chemistry
Asked by vijayvijay09644 | 06 Mar, 2024, 22:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 18 Jul, 2022, 22:39: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 18 Jul, 2022, 22:33: PM
ICSE 10 - Chemistry
Asked by manasa | 10 Sep, 2021, 18:32: PM
ICSE 10 - Chemistry
Asked by waliaman704 | 29 Jun, 2021, 11:58: AM
ICSE 10 - Chemistry
Asked by manbeersinghahhps | 19 May, 2021, 19:12: PM
ICSE 10 - Chemistry
Asked by aras89009 | 09 May, 2021, 14:23: PM
ICSE 10 - Chemistry
Asked by sudhanshutiwari9889 | 29 Sep, 2020, 22:42: PM
ICSE 10 - Chemistry
Asked by adityagogineni15.10spicertl | 17 Jun, 2020, 19:21: PM





