CBSE Class 10 Answered
how methanol is formed from carbon and water
Asked by enterprise.admin1 | 19 Jun, 2019, 21:46: PM
If your question is like this the following is the answer:
1) From carbon in the form of wood:
Methanol is formerly prepared by destructive distillation of wood (Carbon).
1) From carbon in the form of wood:
Methanol is formerly prepared by destructive distillation of wood (Carbon).
2) From water gas: Commercially methanol is formed by a catalytic reaction of a mixture of carbon monoxide (CO) with hydrogen gas (H2) in the presence of the catalyst and under high temperature and pressure. But this mixture of CO and H2 is obtained from the partial burning of coal in the presence of water.
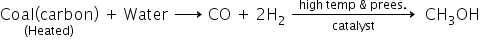
Answered by Ramandeep | 20 Jun, 2019, 15:21: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by agarwalkrishnam98 | 01 Oct, 2023, 08:28: AM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 25 Jan, 2022, 15:01: PM
CBSE 10 - Chemistry
Asked by bhoomikajain68 | 05 Jan, 2022, 21:45: PM
CBSE 10 - Chemistry
Asked by arajeevshashank | 23 Jun, 2020, 16:59: PM
CBSE 10 - Chemistry
Asked by umeshbr824 | 23 Jun, 2020, 06:47: AM
CBSE 10 - Chemistry
Asked by abbabithapm | 23 Nov, 2019, 18:13: PM
CBSE 10 - Chemistry
Asked by gurisingh0908 | 15 Oct, 2019, 16:08: PM
CBSE 10 - Chemistry
Asked by shivanshsoni2167 | 05 Sep, 2019, 09:12: AM
CBSE 10 - Chemistry
Asked by enterprise.admin1 | 19 Jun, 2019, 21:46: PM
CBSE 10 - Chemistry
Asked by das.subir11 | 24 Feb, 2019, 15:38: PM