CBSE Class 11-science Answered
If the reactants are completely transformed into products, the equilibrium constant would be infinity. The equilibrium constants we actually observe all have finite values, implying that even if the products have a lower free energy than the reactants, some of the latter will always remain when the process comes to equilibrium.
To understand how equilibrium constants relate to ΔG° values, assume that all of the reactants are gases, so that the free energy of gas A, for example, is given at all times by
G A=G∘A+RTlnPA
The free energy change for the reaction is sum of the free energies of the products, minus the sum of free energies of the reactants:
ΔG=GC+GD–GA–GB
To expand each term on the right, we have,
ΔG=(G∘C+RTlnPC)+(G∘D+RTlnPD)–(G∘B+RTlnPB)–(G∘A+RTlnP+A)
We can now express the G∘ terms collectively as ΔG∘, and combine the logarithmic pressure terms into a single fraction
ΔG=ΔG°+RTln(PCPD/PAPB)
which is more conveniently expressed in terms of the reaction quotient Q.
ΔG=ΔG∘+RTlnQ
The free energy G is a quantity that becomes more negative during the course of any natural process. Thus as a chemical reaction takes place, G only falls and will never become more positive. Eventually a point is reached where any further transformation of reactants into products would cause G to increase. At this point G is at a minimum, and no further net change can take place; the reaction is at equilibrium.




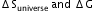 ?
?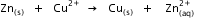 Given that
Given that 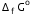 for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.
for Cu2+(aq) and Zn2+(aq) as 65 kJ mol-1 and -147.2 kJ mol-1 respectively.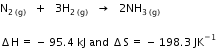 Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.
Calculate the temperature at which Gibbs energy change ΔG is equal to zero. Predict the nature of the reaction at this temperature and above it.