CBSE Class 12-science Answered
How aniline is prepared from (a) benzamide (b) Benzoic acid (c)Nitro benzene
Asked by ap996969 | 16 Mar, 2019, 22:17: PM
(a) From Benzamide:
Amines are prepared by treating an amide with bromine in an aqueous or ethanolic solution of NaOH.In this degradation reaction, migration of alkyl or aryl group takes place from carbonyl carbon of the amide to the nitrogen atom.The amine formed has one carbon atom less than the starting amide.
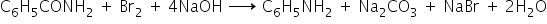
(b)From Benzoic acid:
This conversion takes place in two steps
step 1: benzoic acid to benzamide:
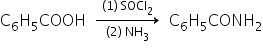
Step 2:
Benzamide to aniline:
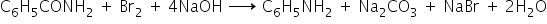
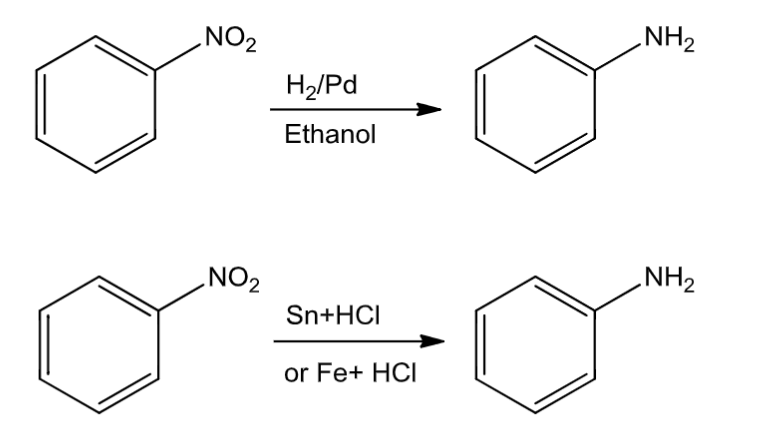
(c) from nitrobenzene:
Nitro compounds on reduction with hydrogen gas in the presence offinely divided nickel, palladium or platinumandon reduction with metals in the acidicmedium give amines.
Answered by Ramandeep | 17 Mar, 2019, 11:34: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 07 Jul, 2022, 20:13: PM
CBSE 12-science - Chemistry
Asked by dhivagar25375 | 12 Aug, 2020, 20:34: PM
CBSE 12-science - Chemistry
Asked by danapalanandhan | 28 Jul, 2020, 11:48: AM
CBSE 12-science - Chemistry
Asked by ap996969 | 16 Mar, 2019, 22:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jul, 2014, 10:07: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jul, 2014, 10:30: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 06 Jun, 2016, 11:13: AM