CBSE Class 11-science Answered
explain kolbe electrolytic method?
Asked by swapnilmishra207 | 07 Jan, 2020, 09:30: PM
The Kolbe reaction is basically a decarboxylative dimerisation and proceeds by a radical reaction mechanism. In this reaction, an aqueous solution of sodium or potassium salt of carboxylic acid is electrolysed wherein dissociation of the salt into carboxylate ion and sodium or potassium ions takes place.
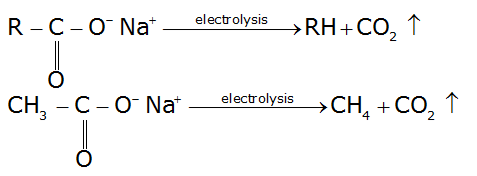
This method is used for the preparation of ethane and the higher alkanes.
Answered by Ramandeep | 08 Jan, 2020, 11:03: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 01:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 01:27: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 22 Jul, 2014, 08:27: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 22 Jul, 2014, 08:29: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 01:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 01 Jun, 2016, 01:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 22 Jul, 2014, 10:04: AM