CBSE Class 9 Answered
Explain distillation method using two separate liquids and lower boiling point difference Acetone and ethanol
Asked by raily.lodh | 11 Nov, 2021, 09:10: AM
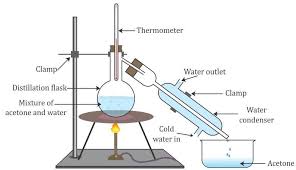
This method is used when the boiling points of two liquids are significantly different from each other or to separate liquids from solids or nonvolatile components. In simple distillation, a mixture is heated to change the most volatile component from a liquid into vapor.
Ethanol is having higher boiling point than acetone so, acetone will form vapour firstly and Ethanol will remain in flask.
Answered by Ravi | 12 Nov, 2021, 13:54: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by aaravsharma10041980 | 06 Dec, 2021, 09:58: AM
CBSE 9 - Chemistry
Asked by raily.lodh | 11 Nov, 2021, 09:10: AM
CBSE 9 - Chemistry
Asked by amirxoku | 03 Jul, 2021, 13:06: PM
CBSE 9 - Chemistry
Asked by dharshini19 | 16 Feb, 2021, 19:29: PM
CBSE 9 - Chemistry
Asked by thevaishnavibaghel | 27 Apr, 2020, 16:28: PM
CBSE 9 - Chemistry
Asked by susmitaroyb | 30 Sep, 2019, 21:02: PM
CBSE 9 - Chemistry
Asked by akshah2407 | 06 Aug, 2019, 18:19: PM
CBSE 9 - Chemistry
Asked by ayushkumarjena07 | 20 Apr, 2019, 19:06: PM
CBSE 9 - Chemistry
Asked by rohit.rt2004 | 21 Feb, 2019, 17:38: PM
CBSE 9 - Chemistry
Asked by Nimmi | 02 Jul, 2018, 11:02: AM





