CBSE Class 11-science Answered
Equal masses of oxygen,hydrogen and methane are taken in a container in identical condition.Find the ratio of volumes of the gases.
Asked by pb_ckt | 29 May, 2019, 11:40: PM
The molecular weights of given mixture of gases are
1. Oxygen = 32
2. Hydrogen = 2
3. Methane = 16
Since masses are equal, assume mass as x,
So By using formula,
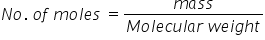 the no. of moles would be,
the no. of moles would be,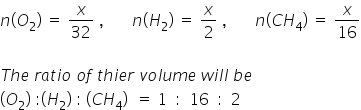
Answered by Ramandeep | 30 May, 2019, 10:38: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




