CBSE Class 11-science Answered
carbon reacts with oxygen to yield CO, depending on quantity of oxygen available per mole of carbon. if excess of O2 is available after formation of CO, then CO2 is produced.
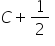
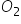
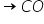
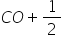
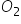
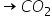
100g of oxygen is reacting with different amounts of carbon as given in the following questions. final out the number of moles of CO andCO2 in each of the follfwing cases.
- 10g of carbon
- 60g of carbon
Asked by Anu | 19 Jun, 2014, 12:43: PM
First reaction C + 1/2 O2 --> CO and second reaction, CO + 1/2 O2 --> CO2
1) For first reaction,
32 g O2 requires 24 g C
100g O2 requires 75 g C
But available C is 10 g, hence C is limiting agent.
12 g C means 1 mole hence 10 g C means 0.833 moles.
Now 1 mole of C produces 1 mole CO
Hence, 0.833 moles of C produces 0.833 moles of CO.
Also from second reaction, 1 mole of CO produces 1 mole of CO2 , hence 0.833 moles of Co produce 0.833 moles of CO2
2) For first reaction,
32 g O2 requires 24 g C
100g O2 requires 75 g C
60 g of carbon is available, hence C is limiting agent.
12 g C means 1 mole hence 60 g C means 5 moles.
As 1 mole of C produces 1 mole CO, hence, 5 moles of C pwill produce 5 moles of CO.
Form second reaction, 1 mole of CO produces 1 mole of CO2
Hence, 5 moles of CO produce 5 moles of CO2
Answered by Prachi Sawant | 20 Jun, 2014, 02:28: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




