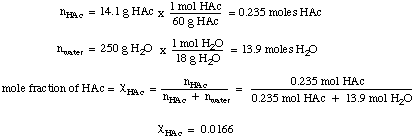CBSE Class 11-science Answered
To calculate the molarity, we find the number of moles of acetic acid, HAc, per liter of solution:
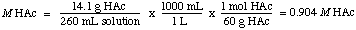
To calculate the molality of the solution, we find the number of moles of acetic acid per kilogram of solvent. Note that we divide by the mass of the solventand not by the mass of the solution.
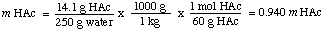
To calculate the mass percent of acetic acid in water we divide the mass of acetic acid, 14.1 g, by the total mass of solution, 264.1 g, and multiply by 100%. The solution is 5.34% acetic acid by mass.
The final concentration calculation is to find the mole fraction of acetic acid in the solution. To do so we find the number of moles of acetic acid, then divide that by the total number of moles in solution: